
Powered Wearable Technologies for Dementia Care: Evaluating
Activity Recognition Models and Dataset Challenges
Mariana Carvalho
1a
, Inês C. Rocha
1b
, Marcelo Arantes
1c
, Ricardo Linhares
1d
,
José Soares
1e
, António Moreira
1,2 f
, João L. Vilaça
1,2 g
, Demétrio Matos
3h
,
Pedro Morais
1,2 i
and Vítor Carvalho
1,2 j
1
2Ai−School of Technology, Polytechnic University of Cávado and Ave (IPCA),
Campus of IPCA, 4750-810 Barcelos, Portugal
2
LASI−Associate Laboratory of Intelligent Systems, 4800-058 Guimarães, Portugal
3
Research Institute for Design, Media and Culture (ID+), School of Design, Polytechnic of Cávado and Ave,
4750-810 Barcelos, Portugal
Keywords: Wearable Technology, Activity Recognition, AI, Elderly, Dementia.
Abstract: Dementia is a progressive neurological condition affecting millions worldwide, posing significant challenges
for patients and caregivers. Wearable technologies integrated with artificial intelligence (AI) provide
promising solutions for continuous activity monitoring, supporting dementia care. This study evaluates the
performance of various AI models, including tree-based methods and deep learning approaches, in
recognizing activities relevant to dementia care. While the first excelled in handling class imbalances and
recognizing common activities, deep learning models demonstrated superior capabilities in capturing complex
temporal and spatial patterns. Additionally, a comprehensive analysis of 30 datasets revealed significant gaps,
including limited representation of elderly participants, insufficient activity coverage, short recording
durations, and a lack of real-world environmental data. To address these gaps, future work should focus on
developing datasets tailored to dementia care, incorporating long-duration recordings, diverse activities, and
realistic contexts. This study highlights the potential of AI-powered wearable systems to transform dementia
management, enabling accurate activity recognition, early anomaly detection, and improved quality of life for
patients and caregivers.
1 INTRODUCTION
Dementia encompasses a range of neurological
disorders characterized by memory loss and cognitive
decline (Winblad et al., 2016). Currently, over 55
million people worldwide live with dementia, and this
number is projected to double by 2050, posing
significant challenges for healthcare systems and
a
https://orcid.org/0009-0004-1818-8213
b
https://orcid.org/0009-0009-7960-7748
c
https://orcid.org/0000-0003-3611-3974
d
https://orcid.org/0009-0001-6108-2463
e
https://orcid.org/0009-0003-3958-7742
f
https://orcid.org/0000-0002-2148-9146
g
https://orcid.org/0000-0002-4196-5357
h
https://orcid.org/0000-0003-4417-6115
i
https://orcid.org/0000-0002-1995-7879
j
https://orcid.org/0000-0003-4658-5844
families (World Health Organization, 2023). With
aging populations and no cure available, the
prevalence of dementia continues to rise.
As the condition progresses, symptoms may
include disorientation, mood swings, confusion,
severe memory loss, behavioural changes, and
difficulties with speaking, swallowing, or walking
(Lindeza et al., 2024). These challenges place a
Carvalho, M., Rocha, I. C., Arantes, M., Linhares, R., Soares, J., Moreira, A., Vilaça, J. L., Matos, D., Morais, P. and Carvalho, V.
Powered Wearable Technologies for Dementia Care: Evaluating Activity Recognition Models and Dataset Challenges.
DOI: 10.5220/0013396600003911
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 18th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2025) - Volume 2: HEALTHINF, pages 995-1006
ISBN: 978-989-758-731-3; ISSN: 2184-4305
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
995

significant emotional and physical burden on both
individuals and their caregivers, requiring substantial
support from an early stage.
The role of caregivers is fundamental in
supporting elderly individuals with dementia.
However, it often presents significant challenges,
including high rates of depression and stress, physical
strain, and social isolation (Lavretsky, 2005). To
address these challenges, enhancing caregiver
support and implementing effective dementia
monitoring are crucial.
Dementia monitoring offers numerous benefits. It
helps prevent accidents by tracking movements and
reducing risks associated with wandering. Enables
long-term health tracking, providing valuable data for
caregivers and healthcare professionals to make
informed care and treatment decisions. Furthermore,
monitoring reduces family anxiety by promptly
alerting caregivers to potential issues and facilitates
patient independence, allowing individuals with
dementia to engage safely in activities both indoors
and outdoors (Lin et al., 2008).
Wearable technology plays a pivotal role in
monitoring individuals with dementia by providing
non-invasive, continuous, and objective data on
various physiological and behavioral parameters.
These devices are generally well-accepted by both
patients and caregivers, making them a practical
solution for continuous monitoring (Husebo et al.,
2020).
One notable application is GPS-enabled
wearables, which help monitor mobility patterns and
locate missing individuals with dementia, offering a
non-intrusive way to track movements and prevent
wandering (Cullen et al., 2022). Additionally, these
devices can report detailed mobility outcomes, such as
activity duration, out-of-home movements, and
trajectory patterns (Cullen et al., 2022). They also
provide insights into health indicators specific to
dementia, including lower daily activity levels,
decreased sleep efficiency, and greater circadian
rhythm variability compared to controls(Cote et al.,
2021).
For patients and caregivers, the comfort,
convenience, and affordability of wearable devices
are key priorities. Essential features include long
battery life, water resistance, and an emergency
button, which enhance usability and reliability
(Stavropoulos et al., 2021).
The work presented in this paper is part of a larger
project focused on developing a wearable device
tailored to the unique needs and challenges of
individuals with dementia. A study from this project
highlights a significant gap in the availability of
comprehensive devices, as most existing wearables
fail to provide an integrated solution that includes
activity monitoring (daily activities, daytime and
nighttime patterns, activity and movement trends),
real-time location tracking, fall detection, and SOS
alert systems (Rocha et al., 2024).
The primary objective of this paper is to describe
the available datasets obtained from wrist-worn
wearables and evaluate the best AI architectures for
predicting activities based on this data. This analysis
provides critical insights into selecting and
developing effective solutions for activity
monitoring, which is an essential step toward
enhancing the functionality of wearable devices for
dementia care.
This paper is organized in seven sections. Section
2 presents the state-of-the-art advancements in AI and
wearable technologies for dementia care, focusing on
activity recognition and the challenges of developing
effective models. Section 3 outlines the methodology
employed, including dataset selection, preprocessing
steps, and the AI models evaluated. Section 4
discusses the datasets analysed in this study,
emphasizing sensor types, participant demographics,
recording durations, and recorded activities. Section
5 provides a detailed analysis of model performance
across various datasets, highlighting the strengths and
limitations of different AI approaches. Section 6
discusses the implications of the findings, challenges
encountered, and recommendations for future
research. Finally, Section 7 concludes the paper,
summarizing key insights and proposing directions
for advancing AI-powered wearable technologies in
dementia care.
2 STATE OF THE ART
In the field of dementia care, wearables and Artificial
Intelligence (AI) are becoming increasingly
significant, offering solutions for monitoring (Husebo
et al., 2020), early diagnosis (Godfrey et al., 2019;
Sashima, 2022), and improved quality of life
(Wilmink et al., 2020).
2.1 Activity Recognition in Dementia
Care
Activity Recognition are crucial in improving care for
individuals with dementia. Through the use of
wearable sensors and machine learning algorithms,
these systems provide valuable insights into patients’
daily activities, supporting caregivers in addressing
deficits and improving care delivery (K. J. Kim et al.,
WHC 2025 - Special Session on Wearable HealthCare
996

2009). For instance, deviations from typical behaviour
can be identified by analysing parameters such as
time, location, and activity duration (Gayathri et al.,
2015).
2.2 AI in Activity Recognition
AI plays a pivotal role in processing sensor data,
identifying patterns, and recognizing activities. By
extracting both spatial and temporal features, AI
enhances the accuracy and efficiency of activity
recognition systems (Khan et al., 2022).
AI algorithms can process streaming data in real-
time, enabling dynamic recognition of human
activities. For example, sliding window-based
approaches combined with time-decay factors have
been shown to improve recognition accuracy in
dynamic environments, ensuring reliability even in
complex, real-world scenarios (Krishnan & Cook,
2014).
One key advantage of AI-driven systems is their
ability to identify new or unexpected activities not
encountered during training. This adaptability
enhances the system’s relevance to real-world
conditions, making it better suited for the
unpredictable nature of dementia care (Leite et al.,
2021).
Different AI techniques offer unique benefits, and
their applicability depends on factors like the
complexity of activities, the nature of sensor data, and
the amount of labelled data available.
2.2.1 Machine Learning Techniques
Machine learning (ML), a subset of AI, is widely used
in activity recognition systems for its ability to learn
patterns from data and generalize them to new
scenarios.
Among traditional ML approaches, Support
Vector Machines (SVMs) are particularly effective
for tasks involving well-separated classes, achieving
high accuracy in identifying activities such as
walking, running, and sitting from wearable data (L.
Cheng et al., 2017). Similarly, Random Forest (RF) is
known for its resilience to noise and ability to classify
multiple activities (Badawi et al., 2019), while K-
Nearest Neighbors (KNN) is most suitable for
datasets with fewer classes, working by comparing
feature similarity (Murad & Pyun, 2017). Logistic
Regression, with its computational efficiency and
interpretability, is commonly used in binary
classification tasks such as distinguishing between
active and inactive states in wearable systems(Rabbi
et al., 2021).
When it comes to Deep Learning approaches,
Convolutional Neural Networks (CNNs) are effective
in learning complex patterns from raw data, ideally
for special feature extraction from sensor data (Khan
et al., 2022). Recurrent Neural Networks (RNNs)
such as Long Short-Term Memory (LSTM) networks
can capture temporal dependencies in sequential
activity data (Murad & Pyun, 2017). Gated Recurrent
Units (GRUs), a variation of RNNs, are particularly
effective for wearable time-series data, as they can
predict transitions between complex activities, such
as alternating sitting and standing, based on
accelerometer and gyroscope readings (Erdaş &
Güney, 2021).
Boosting algorithms like Extreme Gradient
Boosting (XGBoost) and Light Gradient Boosting
Machine (LightGBM) have also proven effective for
wearable data. XGBoost is optimized for speed and
scalability, making it suitable for identifying key
sensor contributions and managing missing data in
activity monitoring applications (Ge, 2023). On the
other hand, LightGBM is particularly advantageous
for processing large datasets and handling real-time
data streams, making it an excellent choice for
latency-critical tasks like fall detection and abnormal
movement tracking (S. T. Cheng, 2017).
Each of these techniques offers unique benefits,
and their applicability depends on factors like the
complexity of activities, the nature of sensor data, and
the volume of labelled data available.
2.2.2 Challenges
Wearable devices collected data is often noisy due to
movement artifacts, environmental interference, or
device misplacement. To address this, techniques
such as feature disentanglement are employed to
separate meaningful activity patterns from irrelevant
noise (Su et al., 2022).
While deep learning methods like CNNs and
LSTMs networks have proven effective for activity
recognition, the integration of data from multiple
sensors, such as accelerometers, gyroscopes, and
heart rate monitors, introduces significant
complexity. This fusion increases computational
demands, posing challenges for both model training
and deployment (Nweke et al., 2018).
Another limitation is the difficulty in generalizing
activity recognition models across users with varying
physical characteristics or across different
environmental contexts. This often leads to reduced
performance in real-world applications, highlighting
the need for models that are adaptable to diverse
scenarios (Lara & Labrador, 2013).
Powered Wearable Technologies for Dementia Care: Evaluating Activity Recognition Models and Dataset Challenges
997
Wearables are limited by battery life and
processing power, making energy-efficient AI
models essential for real-time activity recognition,
hybrid ensemble models and feedback-based
adaptive sampling have been proposed to balance
energy efficiency with recognition accuracy (Min &
Cho, 2011).
3 METHODOLOGY
To train AI models for activity recognition, suitable
datasets are essential. For our study, the ideal dataset
includes data from accelerometers, gyroscopes, and
heart rate sensors, as these sensors provide crucial
insights into movement, orientation and physiological
responses. The dataset should feature labelled activity
data to facilitate supervised learning and encompass
range of activities – such as walking, running, laying,
sleeping, eating, and hygiene – that are particularly
relevant to dementia care. Additionally, it is crucial
for the dataset to include diverse participants,
specifically older adults of both genders, as dementia
predominantly affects this demographic. The selected
datasets were used to train various AI models,
including Support Vector Machines (SVM), Random
Forest (RF), K-Nearest Neighbors (KNN),
Convolutional Neural Networks (CNN), Recurrent
Neural Networks (RNN), Long Short-Term Memory
(LSTM), Extreme Gradient Boosting (XGBoost),
Logistic Regression, Light Gradient Boosting
Machine (LightGBM), and Gated Recurrent Units
(GRU). Model performance was evaluated using
metrics such as precision, recall, and F1-score for
each class, along with overall accuracy, macro-
average, weighted-average, and a confusion matrix to
analyse classification outcomes.
4 DATASETS
To develop and evaluate AI models for activity
recognition in dementia care, this study analysed 30
publicly available datasets commonly used in
wearable activity and health monitoring research.
These datasets were selected to explore their
applicability in detecting activities relevant to
dementia, such as walking, eating, sleeping, and fall-
related movements.
4.1 Sensors
In this study, a total of 30 datasets were analysed to
examine the types of sensors utilized for wearable
activity and health monitoring systems.
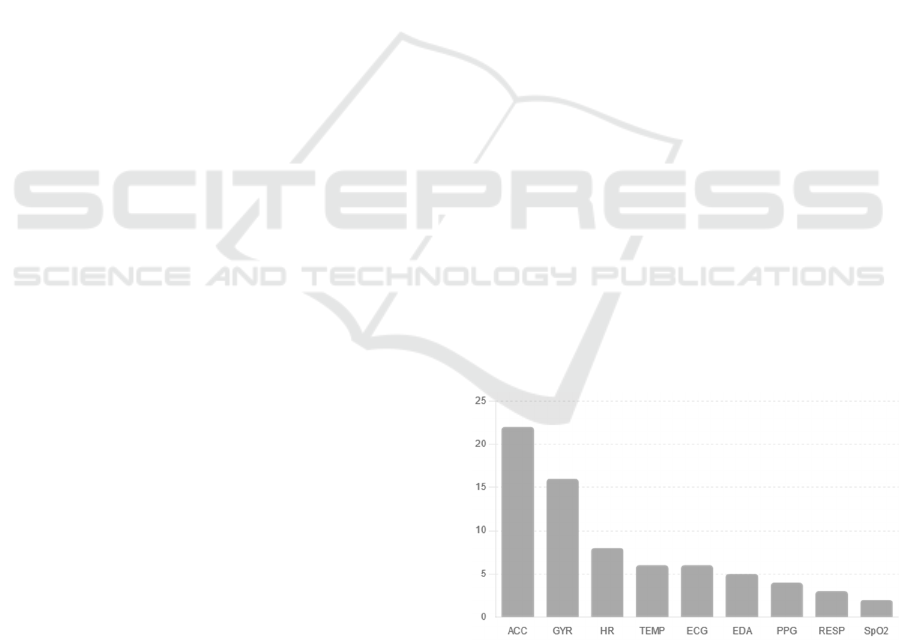
Among these datasets, the most used sensors were
accelerometers (ACC), which appeared in 22 datasets
when combining data from wrist-mounted, chest-
mounted, and general-purpose accelerometers.
Accelerometers are foundational in wearable systems
due to their ability to capture motion data, making
them versatile for applications such as activity
detection, fall monitoring, and posture analysis.
Gyroscopes (GYR) were the second most frequent
sensor type, featured in 16 datasets. These sensors
provide rotational motion data, complementing
accelerometers in capturing more detailed movement
patterns, especially for activities involving complex or
rotational motions.
Heart rate (HR) sensors were present in 8 datasets,
often used for applications requiring cardiovascular
activity tracking.
Other sensors, such as temperature (TEMP)
sensors and electrocardiograms (ECG), were found in
6 datasets each, highlighting their role in physiological
and health monitoring. Electrodermal activity (EDA)
sensors, which measure skin conductance changes and
are used for stress detection, were utilized in 5
datasets. Additionally, respiration (RESP), oxygen
saturation (SpO2), and photoplethysmography (PPG)
sensors were included in a smaller number of datasets,
primarily focusing on health monitoring and specific
physiological applications. Figure 1 provides a visual
representation of the number of datasets utilizing each
sensor type. This analysis underscores the importance
Figure 1: Frequency of Sensor Types Used in the Datasets
- Accelerometer (ACC, m/s²); Gyroscope (GYR, rad/s);
Heart Rate (HR, bpm); Temperature (TEMP, °C);
Electrocardiography (ECG, mV); Electrodermal Activity
(EDA, μS); Photoplethysmogram (PPG); Respiration
(RESP, bpm); Oxygen Saturation (SpO2, %). The vertical
axis represents the count of datasets containing each sensor
type.
WHC 2025 - Special Session on Wearable HealthCare
998
of accelerometers and gyroscopes as fundamental
components in wearable systems for activity
detection. However, the integration of physiological
sensors, such as HR combines physical and health data
to develop more comprehensive monitoring systems.
4.2 Participants Demographics
The demographic composition of participants in
wearable datasets is crucial for developing activity
recognition models tailored to elderly individuals
with dementia. Since dementia predominantly affects
older populations, datasets used for model training
must reflect the physiological and behavioural
characteristics of this demographic. Discrepancies in
age, gender, or participant diversity can lead to
models that fail to generalize effectively to real-world
applications in dementia care.
The total number of participants across datasets
varies significantly. Larger datasets such as the
Parkinsons Disease Smartwatch dataset (PADS)
(Julian et al., 2024), with 469 participants, and the
Sleep Health and Lifestyle Dataset (Tharmalingam,
2023), with 374 participants. In contrast, smaller
datasets like the OPPORTUNITY Activity
Recognition (Roggen, Alberto, et al., 2010), and the
Smartwatch Heart Rate Data Dataset (Biswas &
Ashili, 2023), involve only a single participant.
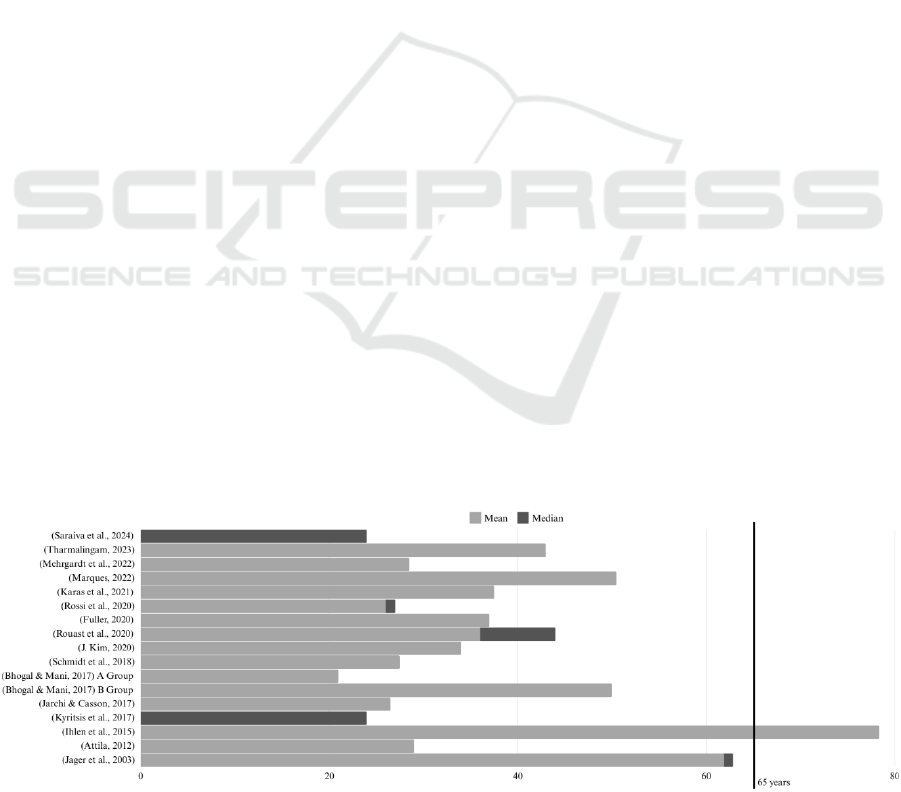
The age range of participants varies, with most of
the datasets focus on adults with a median age of 20-
30 years, some of them being the Physical Activity
Monitoring Dataset PAMAP2 (Attila, 2012),
Objectively Recognizing Eating Behaviour and
Associated Intake (OREBA) (Rouast et al., 2020),
and Annotated Wearable Multimodal Biosignals
recorded during Everyday Life Activities in
Naturalistic Environments (ScientISST MOVE)
(Saraiva et al., 2024), Figure 2.
Datasets targeting specific populations, like the
elderly, include an older demographic. For example,
the Wrist Elderly Daily Activity and Fall Dataset
(WEDA-FALL) (Marques, 2022) has participants
with a mean age of 50.48 years, while the Long-Term
Movement Monitoring Database (Ihlen et al., 2015)
includes participants aged 65–78 years.
Several datasets report near-equal gender
representation. For example, the Sleep Health and
Lifestyle Dataset (Tharmalingam, 2023) has a 51%
male and 49% female split, enhancing model fairness
and applicability across both genders. While others,
such as the Wearable Stress and Affect Detection
(WESAD) (Schmidt et al., 2018), are male
dominated, with only 20% female participants, such
biases may lead to models that underperform for
underrepresented groups.
4.3 Duration of Recordings
To accurately model and monitor daily routines,
datasets must capture a representative snapshot of an
individual's activities throughout the day. Short
recordings may only provide fragmented insights,
while longer recordings enable a view of patterns,
deviations, and anomalies. Extended datasets are
particularly important for identifying changes in
routines, such as prolonged inactivity, increased
wandering, or disruptions in sleep patterns, which are
critical indicators for dementia care.
For example, the Long-Term Movement
Monitoring Database (Ihlen et al., 2015) provides 3
days of continuous data, the Smartwatch heart rate
data (Biswas & Ashili, 2023), includes 1 month of
data, and the Clemson All-day Dataset (CAD)
(Hoover, 2020) spans for 354 days, making these
datasets ideal for tracking routine behaviours over
multiple days.
In contrast, the remaining 25 datasets in the
review capture data for durations shorter than 24
hours, limiting their applicability for in-depth routine
analysis, Figure 3.
Figure 2: Age Distributions in Research Databases.
Powered Wearable Technologies for Dementia Care: Evaluating Activity Recognition Models and Dataset Challenges
999
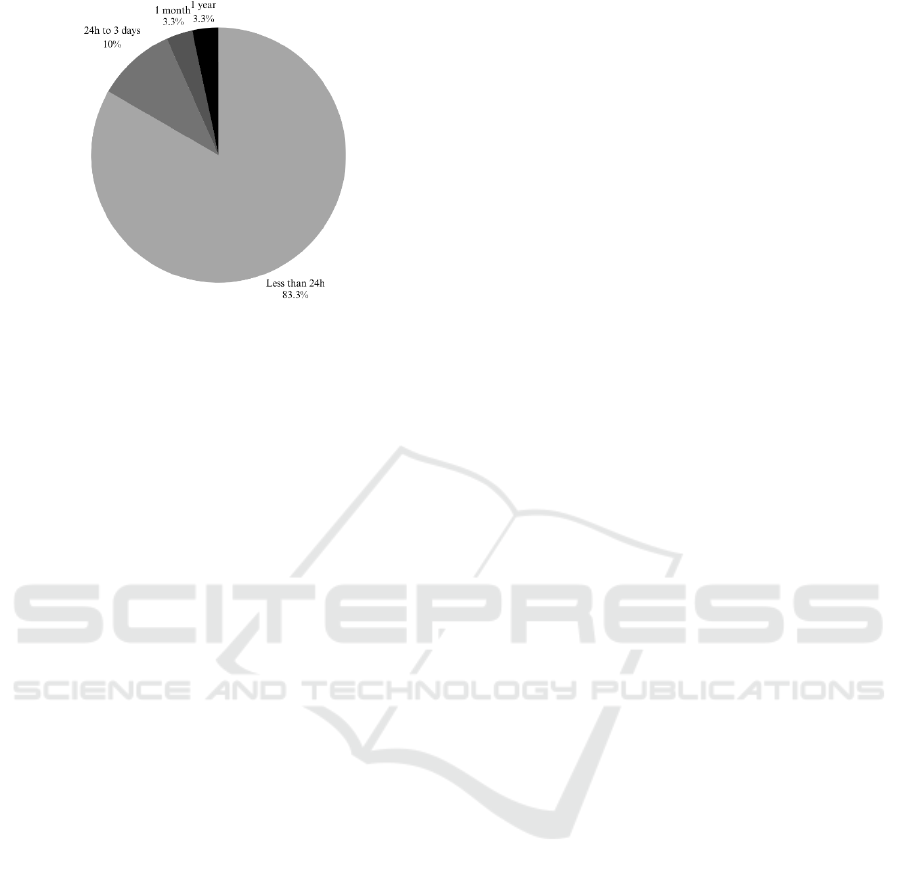
Figure 3: Duration Breakdown of Data Recordings.
4.4 Recorded Activities
Monitoring daily routines requires datasets that
contain a wide range of activities typically performed
throughout the day. This includes basic activities like
walking, sitting, sleeping and eating, as well as more
complex or irregular behaviours such as hygiene
routines, wandering, or falling movements, as seen on
Table 1.
Walking is the most frequently recorded activity
across the datasets, appearing in 40% (12 datasets) of
the reviewed datasets. Eating activities, essential for
monitoring nutritional health and independence, are
labelled in 20% (6 datasets). Sitting and sleeping
activities are recorded in 13.3% (4 datasets) each,
highlighting a focus on sedentary and rest-related
behaviours.
In addition to these activities several datasets (11)
include labels for miscellaneous activities that
provide unique insights into daily routines and
specific behaviours. For instance, the
OPPORTUNITY dataset (Roggen, Alberto, et al.,
2010) includes activities like "opening a door" and
"drinking water," used for recognizing fine-grained
motor skills. The PAMAP2 dataset (Attila, 2012)
features labelled activities such as "ascending stairs",
“descending stairs”, “watching TV”, “standing” and
"house cleaning," capturing more dynamic and
context-specific movements, particularly useful for
training models that aim to recognize household
activities. The WEDA-FALL dataset (Marques,
2022) focuses on fall-related activities and recovery
movements, critical for fall detection systems,
similarly, the Long-Term Movement Monitoring
Database (Ihlen et al., 2015)focuses on prolonged
activity tracking, offering continuous movement data
collected over several days from older adults. Other
datasets, like the ScientISST MOVE dataset (Saraiva
et al., 2024), include transitions between activities
such as "standing-to-sitting" and "sitting-to-lying,"
relevant for understanding changes in posture or
transitions that may indicate health issues. The
OREBA dataset (Rouast et al., 2020) targets eating
behaviours by providing multimodal data for
recognizing eating gestures and associated intake,
contributing to dietary monitoring. The Sleep Health
and Lifestyle Dataset (Tharmalingam, 2023) on the
other hand, focuses on sleep patterns and lifestyle
habits capturing detailed sleep metrics such as
duration, efficiency, and disruptions, which are vital
for understanding circadian rhythm irregularities
often observed in dementia patients.
The WESAD (Schmidt et al., 2018), are focused
on stress recognition, providing labelled data for
different emotional states, including stress,
amusement, and neutral conditions. These datasets
often integrate physiological signals, such as heart
rate variability (HRV), electrodermal activity (EDA),
and respiratory patterns, alongside motion data.
Each of these datasets provides unique insights
and data characteristics that enrich the development
of AI models, enabling more comprehensive and
accurate activity recognition systems tailored to
dementia care.
5 MODEL PERFORMANCE
ANALYSIS
To evaluate the effectiveness of machine learning
models in activity recognition, we analysed the
performance of multiple algorithms across various
datasets. This section summarizes the results obtained
for each dataset. Performance metrics, including
precision, recall, and F1-score, were evaluated for
models like Random Forest, K-Nearest Neighbors
(KNN), and Gradient Boosting.
5.1 MMASH Dataset
The MMASH (Multimodal Activity Recognition in
Smart Home Environments) (Rossi et al., 2020)
dataset is a comprehensive dataset designed for
activity recognition research. It includes data from
multiple sensor types such as accelerometers,
gyroscopes, magnetometers, and physiological
sensors. Covering a wide range of activities, including
basic actions like sitting, walking, and lying down, as
well as complex activities such as eating or
performing household tasks.
WHC 2025 - Special Session on Wearable HealthCare
1000
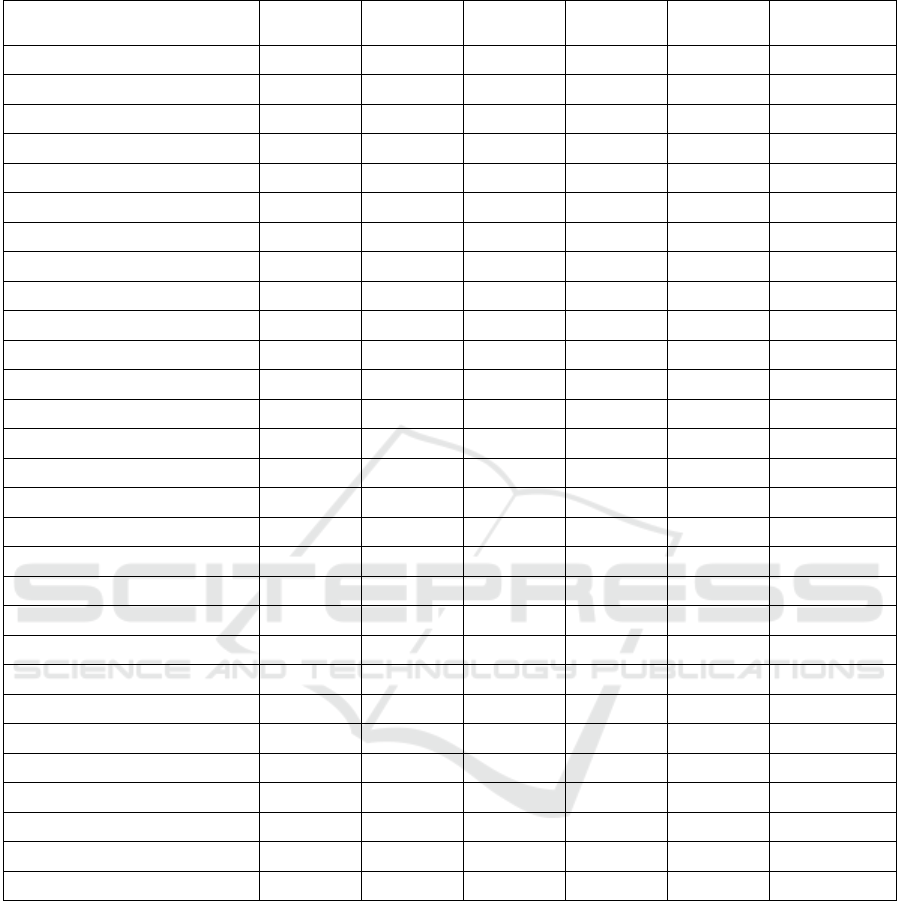
Table 1: Activity Types Captured in Wearable Activity Recognition Datasets.
Ref./ Activity Walk Sit Sleep Eat Fall Miscellaneous
(Saraiva et al., 2024) x x
(Guy et al., 2024) x
(Nicoomanesh, 2024) x x
(Julian et al., 2024)
(Godzwon, 2024)
(Tharmalingam, 2023) x x x
(Grimaldi et al., 2023) x x x
(Mehrgardt et al., 2022)
(Amin et al., 2022)
(Marques, 2022) x x x
(Karas et al., 2021) x x
(Rossi et al., 2020) x x x x x
(Fuller, 2020) x x x
(Hoover, 2020) x
(Rouast et al., 2020) x
(J. Kim, 2020) x x
(Walch, 2019) x x
(Kyritsis et al., 2019) x
(Schmidt et al., 2018)
(Jafarnejad, 2018)
(Bhogal & Mani, 2017)
(Jarchi & Casson, 2017) x x
(Kyritsis et al., 2017) x
(Ihlen et al., 2015) x
(Banos et al., 2014) x x x x x
(Attila, 2012) x x
(Roggen, Calatroni, et al., 2010)
(Jager et al., 2003)
(Moody & Mark, 2001)
Both the XGBoost and LigthGBM models
consistently achieved higher accuracy, and F1-scores
compared to other models. For instance, the
XGBoost, demonstrated strong generalization with
higher overall accuracy and consistently balanced
precision and recall across activities, including
underrepresented classes. With the LightGBM
outperforming in handling imbalanced data,
particularly for rare activities.
The Random Forest and Gradient Boosting
models performed best for the generalized activities
with large support values, such as sitting. However,
the model struggled with specific or underrepresented
activities such as large screen usage and sleeping.
Comparing to other models, the KNN
underperformed, presenting low precision and recall
values for most activities, due to the class imbalance.
5.2 ScientISST Dataset
The ScientISST Dataset (Saraiva et al., 2024) is a
comprehensive and multimodal dataset designed for
human activity and gesture recognition. It is
particularly valuable for developing and evaluating
machine learning models in scenarios requiring high
Powered Wearable Technologies for Dementia Care: Evaluating Activity Recognition Models and Dataset Challenges
1001

precision and robustness, such as healthcare,
wearable technology, and human-computer
interaction.
The KNN and Random Forest models
outperformed the other models, achieving nearly
perfect results across most activities for accuracy,
precision and F1-score, apart from the less frequent
activities.
The CNN and GRU models performed well in
recognizing frequent and sequential activities. CNN
excelled at extracting spatial features, achieving high
F1-scores for structured tasks like "Run" (0.95) and
dynamic movements like "Jumps" (0.74). GRU
effectively captured temporal dependencies, making
it ideal for activities with transitions, such as "Lift"
(F1-score: 0.83) and "Run" (F1-score: 0.93). The
SVM model showed strong performance for well-
separated and frequent activities. The MLP model
demonstrated consistent performance for frequent
and distinct activities, achieving an F1-score of 0.97
for "Run."
All models, however, struggled with nuanced
gestures like "Greetings" and “jump” presenting
reduced precision and recall.
5.3 PAMAP2 Dataset
The PAMAP2 (Physical Activity Monitoring for
Aging People 2) (Attila, 2012) dataset is a collection
of data designed to facilitate the development and
evaluation of activity recognition algorithms. This
dataset is widely utilized in the field of wearable
computing and health monitoring, particularly for
applications involving elderly care and fitness
tracking.
The Random Forest and XGBoost models exhibit
stellar performance, with nearly perfect precision,
recall, and F1-scores close to 100% across a wide
range of activities. This performance indicates their
robust predictive capabilities and adaptability in
managing diverse data types, particularly in complex
activity scenarios such as 'nordic walking' and
'cycling'.
In a similar way, LightGBM, a gradient boosting
model optimized for speed and reduced memory
usage, offers substantial advantages for real-time
activity recognition applications. Combining the
robust framework of gradient boosting with
enhancements designed to improve processing speed
and efficiency, making it competitive for applications
where quick response times are crucial.
The KNN model showed moderate performance
with an overall accuracy of 91%. While it performed
well on frequent activities like "Sitting" (F1-score:
0.98) and "Cycling" (F1-score: 0.96), its performance
dropped for more complex and underrepresented
activities, such as "Ascending Stairs" (F1-score: 0.74)
and "Descending Stairs" (F1-score: 0.72).
The Logistic Regression model shows varying
performance across different activities, reflecting
some fundamental limitations in handling complex,
multiclass problems. While it performs commendably
in activities with clear distinctions, such as 'lying' and
'ironing', it faces challenges in activities requiring
nuanced differentiation, such as 'standing' versus
'sitting'. This variation highlights the need for
sophisticated feature engineering or advanced data
preprocessing to bolster its effectiveness in more
complex scenarios.
6 DISCUSSION
The findings of this study provide important insights
into the development and optimization of AI models
and wearable technologies for activity recognition in
dementia care. However, a significant challenge is the
lack of comprehensive datasets tailored to the unique
requirements of this domain. Current datasets
predominantly feature younger adults, offering
limited representation of older individuals who are
most affected by dementia, thereby reducing the
applicability of AI models to the intended population.
Additionally, existing datasets often fail to cover the
full range of activities relevant to dementia care, such
as hygiene routines, eating behaviors, wandering, and
fall-related movements. This lack of comprehensive
activity coverage limits the ability of AI systems to
monitor the complex behaviors associated with
dementia effectively.
Another limitation is the prevalence of short-
duration recordings, which are insufficient for
analyzing long-term activity patterns and deviations
that are critical for dementia monitoring and detecting
changes in routine or health status. Furthermore, most
datasets are collected in controlled environments,
which fail to capture the complexity and variability of
real-world settings, such as homes or assisted living
facilities where dementia patients typically reside.
This discrepancy reduces the robustness and
generalizability of models trained on such data.
Additionally, many datasets suffer from significant
class imbalances, with underrepresented activities
leading to poor model performance for these specific
behaviors. Addressing these limitations is essential to
develop AI-driven wearable solutions that are
accurate, robust, and capable of meeting the practical
needs of dementia care.
WHC 2025 - Special Session on Wearable HealthCare
1002

Future datasets should prioritize the inclusion of
elderly participants representing diverse genders and
cognitive stages, ensuring that the data accurately
reflects the population most affected by dementia.
These datasets should aim to capture a comprehensive
range of activities, including eating, hygiene routines,
wandering, fall recovery, and activity transitions, as
well as nighttime behaviours. To enable a deeper
understanding of daily routines and their variations, it
is essential to include long-duration recordings
spanning multiple days or even weeks. Collecting
data in naturalistic environments, such as homes or
care facilities, will further enhance the validity of the
datasets and significantly improve the robustness and
generalizability of AI models developed for dementia
care.
The performance evaluation of the AI models in
this study highlights the strengths and limitations of
different approaches for activity recognition in
dementia care. Models such as Random Forest (RF),
XGBoost, and LightGBM consistently demonstrated
robust performance, excelling in handling class
imbalances and accurately recognizing well-defined
activities like walking, running, and sitting. Their
resilience to noisy data and ability to generalize
across common activity classes make them reliable
choices for general activity monitoring.
However, despite these promising results, all
models faced challenges in identifying less frequent
and more nuanced activities, such as eating or
transitions, due to limitations in dataset quality and
class imbalances. The underrepresentation of these
critical activities in the datasets hindered model
performance, leading to reduced precision and recall
for these classes. Moreover, the prevalence of short-
duration recordings further constrained the models'
ability to analyze long-term activity patterns, limiting
their effectiveness in detecting behavioral trends and
anomalies essential for dementia care.
These findings underscore the necessity of
selecting and tailoring models based on specific
application requirements. For general activity
recognition tasks, tree-based models like XGBoost
and LightGBM offer strong performance and
efficiency. In contrast, deep learning approaches,
such as CNNs and GRUs, are better suited for tasks
that require detailed temporal and spatial analysis,
particularly when handling complex or transitional
activities. Addressing dataset limitations, including
activity coverage, class balance, and recording
duration, will be critical for enhancing model
performance and ensuring their practical applicability
in real-world dementia care scenarios.
7 CONCLUSION
This study highlights the potential of AI models and
wearable technologies for activity recognition in
dementia care, demonstrating the strengths of tree-
based models like Random Forest, XGBoost, and
LightGBM in handling class imbalances and
recognizing common activities, as well as the
capabilities of deep learning models such as CNNs
and GRUs in capturing complex patterns and
transitions. However, significant challenges remain,
including the lack of comprehensive datasets that
adequately represent the elderly population,
encompass a diverse range of activities, and provide
long-duration recordings in real-world environments.
These limitations reduce the generalizability and
effectiveness of AI models in detecting nuanced
behaviors and long-term activity patterns critical for
dementia monitoring.
To address these gaps, future research should
focus on developing tailored datasets with enhanced
demographic diversity, extended recordings, and
realistic environmental contexts. Combining
traditional and deep learning models into hybrid
approaches can further optimize performance, while
energy-efficient AI solutions will ensure real-time
monitoring capabilities for wearable devices. By
overcoming these challenges, AI-powered wearable
technologies can play a transformative role in
dementia care, enabling accurate activity recognition,
early intervention, and improved quality of life for
patients while reducing the burden on caregivers.
ACKNOWLEDGEMENTS
This project was funded through the Foundation for
Science and Technology (FCT) under the projects
UIDB/05549/2020(DOI:10.54499/UIDB/05549/202
0), UIDP/05549/2020 (DOI:10.54499/UIDP/05549/
2020), LASILA/P/0104/2020, and CEECINST/
00039/2021. This work was also funded by the
Innovation Pact HfFP–Health From Portugal, co-
funded from the” Mobilizing Agendas for Business
Innovation” of the ”Next Generation EU” program of
Component 5 of the Recovery and Resilience Plan
(RRP), concerning ”Capitalization and Business
Innovation”, under the Regulation of the Incentive
System ”Agendas for Business Innovation”.
Powered Wearable Technologies for Dementia Care: Evaluating Activity Recognition Models and Dataset Challenges
1003

REFERENCES
Amin, M. R., Wickramasuriya, D., & Faghih, R. T. (2022).
A Wearable Exam Stress Dataset for Predicting
Cognitive Performance in Real-World Settings
[Dataset]. PhysioNe. https://doi.org/https://doi.org/
10.13026/kvkb-aj90
Attila, R. (2012). PAMAP2 Physical Activity Monitoring
[Dataset]. UCI Machine Learning Repository.
https://doi.org/https://doi.org/10.24432/C5NW2H.
Badawi, A. A., Al-Kabbany, A., & Shaban, H. (2019).
Multimodal Human Activity Recognition from
Wearable Inertial Sensors Using Machine Learning.
402–407. https://doi.org/10.1109/iecbes.2018.8626737
Banos, O., Garcia, R., Holgado-Terriza, J. A., Damas, M.,
Pomares, H., Rojas, I., Saez, A., & Villalonga, C.
(2014). mHealthDroid: A Novel Framework for Agile
Development of Mobile Health Applications.
Bhogal, A. S., & Mani, A. R. (2017). Pattern analysis of
oxygen saturation variability in healthy individuals:
Entropy of pulse oximetry signals carries information
about mean oxygen saturation. Frontiers in Physiology,
8(AUG). https://doi.org/10.3389/fphys.2017.00555
Biswas, N., & Ashili, S. (2023). Smartwatch heart rate
data. IEEE Dataport. https://doi.org/https://dx.doi.org/
10.21227/y4ek-d516
Cheng, L., Guan, Y., Zhu, K., & Li, Y. (2017). Recognition
of Human Activities using Machine Learning Methods
with Wearable Sensors. IEEE.
Cheng, S. T. (2017). Dementia Caregiver Burden: a
Research Update and Critical Analysis. In Current
Psychiatry Reports (Vol. 19, Issue 9). Current Medicine
Group LLC 1. https://doi.org/10.1007/s11920-017-
0818-2
Cote, A. C., Phelps, R. J., Kabiri, N. S., Bhangu, J. S., &
Thomas, K. (2021). Evaluation of Wearable
Technology in Dementia: A Systematic Review and
Meta-Analysis. In Frontiers in Medicine (Vol. 7).
Frontiers Media S.A. https://doi.org/10.3389/
fmed.2020.501104
Cullen, A., Mazhar, M. K. A., Smith, M. D., Lithander, F.
E., Breasail, M., & Henderson, E. J. (2022). Wearable
and Portable GPS Solutions for Monitoring Mobility in
Dementia: A Systematic Review. Sensors, 22(9).
https://doi.org/10.3390/s22093336
Erdaş, Ç. B., & Güney, S. (2021). Human Activity
Recognition by Using Different Deep Learning
Approaches for Wearable Sensors. In Neural
Processing Letters (Vol. 53, Issue 3, pp. 1795–1809).
Springer. https://doi.org/10.1007/s11063-021-10448-3
Fuller, D. (2020). Replication Data for: Using machine
learning methods to predict physical activity types with
Apple Watch and Fitbit data using indirect calorimetry
as the criterion. Harvard Dataverse.
https://doi.org/https://doi.org/10.7910/DVN/ZS2Z2J
Gayathri, K. S., Elias, S., & Ravindran, B. (2015).
Hierarchical activity recognition for dementia care
using Markov Logic Network. Personal and
Ubiquitous Computing, 19(2), 271–285.
https://doi.org/10.1007/s00779-014-0827-7
Ge, R. (2023). XGBoost-Based Human Activity
Recognition Algorithm using Wearable Smart Devices.
Applied and Computational Engineering, 2(1), 352–
358. https://doi.org/10.54254/2755-2721/2/20220514
Godfrey, A., Brodie, M., van Schooten, K. S.,
Nouredanesh, M., Stuart, S., & Robinson, L. (2019).
Inertial wearables as pragmatic tools in dementia. In
Maturitas (Vol. 127, pp. 12–17). Elsevier Ireland Ltd.
https://doi.org/10.1016/j.maturitas.2019.05.010
Godzwon, I. (2024). FitBit Heart Rate [Dataset]. OpenML.
https://www.openml.org/search?type=data&status=act
ive&id=46103&sort=runs
Grimaldi, E., Vigneri, D., Di Poce, G., & Grieco, N. (2023).
Falls vs Normal Activities [Dataset]. Kaggle.
https://www.kaggle.com/datasets/enricogrimaldi/falls-
vs-normal-activities
Guy, E. F. S., Isaac, F., Jaimey Anne, C., Trudy, C. der K.,
Rongqing, C., Jennifer, K., Knut, M., & James
Geoffrey, C. (2024). Respiratory and heart rate
monitoring dataset from aeration study [Dataset]].
PhysioNet. https://doi.org/https://doi.org/10.13026/
e4dt-f689
Hoover, A. (2020). Clemson All-day Dataset (CAD)
[Dataset]. Clemson University. https://cecas.
clemson.edu/~ahoover/allday/
Husebo, B. S., Heintz, H. L., Berge, L. I., Owoyemi, P.,
Rahman, A. T., & Vahia, I. V. (2020). Sensing
technology to facilitate behavioral and psychological
symptoms and to monitor treatment response in people
with dementia: A systematic review. In Frontiers in
Pharmacology (Vol. 10). Frontiers Media S.A.
https://doi.org/10.3389/fphar.2019.01699
Ihlen, E. A. F., Weiss, A., Helbostad, J. L., & Hausdorff, J.
M. (2015). The Discriminant Value of Phase-
Dependent Local Dynamic Stability of Daily Life
Walking in Older Adult Community-Dwelling Fallers
and Nonfallers. BioMed Research International, 2015,
1–11. https://doi.org/10.1155/2015/402596
Jafarnejad, S. (2018). An Open Dataset for Human Activity
Analysis [Dataset]. Kaggle. https://www.kaggle.com/
datasets/sasanj/human-activity-smart-devices
Jager, F., Taddei, A., Moody, G. B., Emdin, M., Antolic,
G., Dorn, R., Smrdl, A., Marchesi, C., & Mark, R. G.
(2003). Long Term ST Database . PhysioNet.
Jarchi, D., & Casson, A. J. (2017). Description of a database
containing wrist PPG signals recorded during physical
exercise with both accelerometer and gyroscope
measures of motion. Data, 2(1). https://doi.org/
10.3390/data2010001
Julian, V., Alexander, B., Lucas, P., Catharina, van A.,
Michael, F., & Tobias, W. (2024). PADS - Parkinsons
Disease Smartwatch dataset. PhysioNet.
Karas, M., Urbanek, J., Crainiceanu, C., Harezlak, J., &
Fadel, W. (2021). Labeled raw accelerometry data
captured during walking, stair climbing and driving
[Dataset]. PhysioNet. https://doi.org/10.13026/51h0-
a262
Khan, I. U., Afzal, S., & Lee, J. W. (2022). Human activity
recognition via hybrid deep learning based model.
Sensors, 22(1). https://doi.org/10.3390/s22010323
WHC 2025 - Special Session on Wearable HealthCare
1004

Kim, J. (2020). UCI-HAR [Dataset]. Kaggle.
https://kaggle.com/competitions/uci-har
Kim, K. J., Hassan, M. M., Na, S., & Huh, E. N. (2009).
Dementia wandering detection and activity recognition
algorithm using tri-axial accelerometer sensors.
Proceedings of the 4th International Conference on
Ubiquitous Information Technologies and
Applications, ICUT 2009. https://doi.org/
10.1109/ICUT.2009.5405672
Krishnan, N. C., & Cook, D. J. (2014). Activity recognition
on streaming sensor data. Pervasive and Mobile
Computing, 10(PART B), 138–154.
https://doi.org/10.1016/j.pmcj.2012.07.003
Kyritsis, K., Diou, C., & Delopous, A. (2017). Wrist-
mounted IMU data towards the investigation of in-meal
human eating behavior - the Food Intake Cycle (FIC)
dataset. IEEE Journal of Biomedical and Health
Informatics.
Kyritsis, K., Diou, C., & Delopous, A. (2019). Wrist-
mounted IMU data towards the investigation of free-
living human eating behavior - the Free-living Food
Intake Cycle (FreeFIC) dataset. NIAID Data
Ecosystem. https://doi.org/10.5281/zenodo.4421860
Lara, Ó. D., & Labrador, M. A. (2013). A survey on human
activity recognition using wearable sensors. IEEE
Communications Surveys and Tutorials, 15(3), 1192–
1209. https://doi.org/10.1109/
SURV.2012.110112.00192
Lavretsky, H. (2005). Stress and Depression in Informal
Family Caregivers of Patients with Alzheimer’s
Disease. Aging Health, 1(1), 117–133.
https://doi.org/10.2217/1745509x.1.1.117
Leite, B., Abdalrahman, A., Castro, J., Frade, J., Moreira,
J., & Soares, C. (2021). Novelty detection in physical
activity. ICAART 2021 - Proceedings of the 13th
International Conference on Agents and Artificial
Intelligence, 2, 859–865. https://doi.org/10.5220/
0010254908590865
Lin, C. C., Lin, P. Y., Lu, P. K., Hsieh, G. Y., Lee, W. L.,
& Lee, R. G. (2008). A healthcare integration system
for disease assessment and safety monitoring of
dementia patients. IEEE Transactions on Information
Technology in Biomedicine, 12(5), 579–586.
https://doi.org/10.1109/TITB.2008.917914
Lindeza, P., Rodrigues, M., Costa, J., Guerreiro, M., &
Rosa, M. M. (2024). Impact of dementia on informal
care: a systematic review of family caregivers’
perceptions. In BMJ Supportive and Palliative Care
(Vol. 14, Issue e1, pp. E38–E49). BMJ Publishing
Group. https://doi.org/10.1136/bmjspcare-2020-
002242
Marques, J. (2022). Wrist Elderly Daily Activity and Fall
Dataset (WEDA-FALL).
Mehrgardt, P., Khushi, M., Poon, S., & Withana, A. (2022).
Pulse Transit Time PPG Dataset. PhysioNet.
https://doi.org/https://doi.org/10.13026/jpan-6n92
Min, J.-K., & Cho, S.-B. (2011). Activity Recognition based
on Wearable Sensors Using Selection/Fusion Hybrid
Ensemble. IEEE.
Moody, G. B., & Mark, R. G. (2001). The Impact of the
MIT-BIH Arrhythmia Database History, Lessons
Learned, and Its Influence on Current and Future
Databases.
Murad, A., & Pyun, J. Y. (2017). Deep recurrent neural
networks for human activity recognition. Sensors
(Switzerland), 17(11). https://doi.org/10.3390/
s17112556
Nicoomanesh, A. (2024). FitBit Fitness Tracker Data
[Dataset]. Kaggle. https://www.kaggle.com/datasets/
arashnic/fitbit/data
Nweke, H. F., Teh, Y. W., Al-garadi, M. A., & Alo, U. R.
(2018). Deep learning algorithms for human activity
recognition using mobile and wearable sensor
networks: State of the art and research challenges. In
Expert Systems with Applications (Vol. 105, pp. 233–
261). Elsevier Ltd.
https://doi.org/10.1016/j.eswa.2018.03.056
Rabbi, J., Fuad, Md. T. H., & Awal, Md. A. (2021). Human
Activity Analysis and Recognition from Smartphones
using Machine Learning Techniques. http://arxiv.org/
abs/2103.16490
Rocha, I. C., Arantes, M., Moreira, A., Vilaça, J. L., Morais,
P., Matos, D., & Carvalho, V. (2024). Monitoring
Wearable Devices for Elderly People with Dementia: A
Review. In Designs (Vol. 8, Issue 4). Multidisciplinary
Digital Publishing Institute (MDPI).
https://doi.org/10.3390/designs8040075
Roggen, D., Alberto, C., Long-Van, N.-D., Ricardo, C., &
Hesam, S. (2010). OPPORTUNITY Activity
Recognition [Dataset]. UCI Machine Learning
Repository. https://doi.org/10.24432/C5M027.
Roggen, D., Calatroni, A., Rossi, M., Holleczek Thomas,
Forster, K., Troster, G., Lukowicz, P., Bannach, D., &
Pirkl, G. (2010). Collecting complex activity datasets in
highly rich networked sensor environments.
Rossi, A., Da Pozzo, E., Menicagli, D., Tremolanti, C.,
Priami, C., Sirbu, A., Clifton, D., Martini, C., &
Morelli, D. (2020). Multilevel Monitoring of Activity
and Sleep in Healthy People [Database]]. PhysioNet.
https://doi.org/https://doi.org/10.13026/cerq-fc86
Rouast, P. V., Heydarian, H., Adam, M. T. P., & Rollo, M.
E. (2020). OReBA: A dataset for objectively
recognizing eating behavior and associated intake.
IEEE Access, 8, 181955–181963.
https://doi.org/10.1109/ACCESS.2020.3026965
Saraiva, J. A., Abreu, M., Carmo, A. S., Silva, H. P., &
Fred, A. (2024). ScientISST MOVE: Annotated
Wearable Multimodal Biosignals recorded during
Everyday Life Activities in Naturalistic Environments.
PhysioNet. https://doi.org/10.13026/hyxq-r919.
Sashima, A. (2022). Machine-learning-driven Wearable
Healthcare for Dementia: A Review of Emerging
Technologies and Challenges. 864–871.
https://doi.org/10.5220/0010973900003123
Schmidt, P., Reiss, A., Duerichen, R., & Van Laerhoven, K.
(2018). Introducing WeSAD, a multimodal dataset for
wearable stress and affect detection. ICMI 2018 -
Proceedings of the 2018 International Conference on
Powered Wearable Technologies for Dementia Care: Evaluating Activity Recognition Models and Dataset Challenges
1005

Multimodal Interaction, 400–408. https://doi.org/
10.1145/3242969.3242985
Stavropoulos, T. G., Lazarou, I., Diaz, A., Gove, D.,
Georges, J., Manyakov, N. V., Pich, E. M., Hinds, C.,
Tsolaki, M., Nikolopoulos, S., & Kompatsiaris, I.
(2021). Wearable Devices for Assessing Function in
Alzheimer’s Disease: A European Public Involvement
Activity About the Features and Preferences of Patients
and Caregivers. Frontiers in Aging Neuroscience, 13.
https://doi.org/10.3389/fnagi.2021.643135
Su, J., Wen, Z., Lin, T., & Guan, Y. (2022). Learning
disentangled behaviour patterns for wearable-based
human activity recognition. Proceedings of the ACM on
Interactive, Mobile, Wearable and Ubiquitous
Technologies, 6(1). https://doi.org/10.1145/3517252
Tharmalingam, L. (2023). Sleep Health and Lifestyle
Dataset. Kaggle. https://www.kaggle.com/datasets/
uom190346a/sleep-health-and-lifestyle-dataset/data
Walch, O. (2019). Motion and heart rate from a wrist-worn
wearable and labeled sleep from polysomnography
[Database]. PhysioNet. https://doi.org/https://doi.org/
10.13026/hmhs-py35
Wilmink, G., Dupey, K., Alkire, S., Grote, J., Zobel, G.,
Fillit, H. M., & Movva, S. (2020). Artificial
intelligence-powered digital health platform and
wearable devices improve outcomes for older adults in
assisted living communities: Pilot intervention study.
JMIR Aging, 3(2). https://doi.org/10.2196/19554
Winblad, B., Amouyel, P., Andrieu, S., Ballard, C., Brayne,
C., Brodaty, H., Cedazo-Minguez, A., Dubois, B.,
Edvardsson, D., Feldman, H., Fratiglioni, L., Frisoni,
G. B., Gauthier, S., Georges, J., Graff, C., Iqbal, K.,
Jessen, F., Johansson, G., Jönsson, L., Zetterberg, H.
(2016). Defeating Alzheimer’s disease and other
dementias: A priority for European science and society.
In The Lancet Neurology (Vol. 15, Issue 5, pp. 455–
532). Lancet Publishing Group. https://doi.org/
10.1016/S1474-4422(16)00062-4
WHC 2025 - Special Session on Wearable HealthCare
1006
