
Millimeter-Wave Systems for Real-Time Intraoperative Brain Tumor
Resection Assistance
H. Lopes
1,2 a
, P. M. Mendes
1,2 b
and H. Dinis
1,2 c
1
Center for MicroElectromechanical Systems (CMEMS-Uminho), University of Minho, 4800-058 Guimarães, Portugal
2
pg53861@alunos.uminho.pt, {pmendes, hdinis}@dei.uminho.pt
Keywords: Brain Cancer, Brain Tumor Detection, Microwave Imaging, mmWave, Dielectric Properties, Real-Time
Surgical Assistance.
Abstract: Brain cancer is one of the deadliest forms of cancer due to limited treatment options and challenges in tumor
differentiation during surgery. Current surgical assistance tools, such as intraoperative imaging systems and
advanced visualization techniques, often face limitations in cost, accessibility, and precision. Microwave and
millimeter-wave (mmWave) technologies have emerged as promising alternatives for real-time, non-invasive
differentiation of healthy and cancerous brain tissues, leveraging their sensitivity to dielectric property
variations. This paper reviews the state-of-the-art microwave and mmWave systems developed for medical
diagnostics, focusing on brain tumor detection. It highlights their underlying principles, performance, and
limitations while discussing their potential to address the drawbacks of existing tools. By analyzing recent
advancements, the review identifies key areas for future development, proposing characteristics of an ideal
system to support real-time surgical decision-making. Additionally, the paper proposes a system designed to
measure the dielectric properties of the brain tissue, aiming to enhance real-time surgical decision-making
and improve patient outcomes.
1 INTRODUCTION
The last GLOBOCAN report showed that in 2022 there
were nearly 20 million new cancer cases and that
almost 10 million deaths were caused by it, meaning
that one in five individuals can potentially develop
cancer throughout their lives. Despite ranking
nineteenth in new cases, brain cancer has limited
treatment options available, making it one of the most
devastating prognoses, often fatal (Bray et al., 2024).
According to the available data, brain cancer has
a 5-year relative survival rate of 33.4%, which drops
to less than 10% for the most aggressive brain tumors,
such as glioblastomas (Brain and Other Nervous
System Cancer — Cancer Stat Facts, 2020). To try
and help cure the brain tumor, as of today, the main
treatments used are radiotherapy and surgery,
craniotomy being the most common surgery in this
case. In this surgery, a neurosurgeon cuts out an area
of bone from the skull exposing the dura mater. After
a
https://orcid.org/0009-0002-0019-1591
b
https://orcid.org/0000-0003-2177-7321
c
https://orcid.org/0000-0002-2394-2119
the cut and removal of this tissue, a part of the brain
is exposed, and the resection of the tumor is possible
(Surgery for Brain Tumours - Cancer Research UK,
2023).
Despite being an area in constant evolution,
surgery is often insufficient to provide a permanent
cure for some of the most aggressive tumors, like
high-grade gliomas and medulloblastomas (Delaidelli
& Moiraghi, 2024). Many times it is not possible to
remove the full mass of the cancer leaving behind
some parts of it, given that distinguishing the tumor
from the surrounding healthy tissue is not an easy task
(Delaidelli & Moiraghi, 2024; Surgery for Brain
Tumours - Cancer Research UK, 2023). Usually, the
surgeon has to use expertise and previous knowledge
to decide if the tissue is healthy or malignant. Studies
show that 80% to 90% of tumor recurrence has origin
in an incomplete resection (Petrecca et al., 2013).
Even though the diagnostic techniques for brain
tumor detection have an extended characterization,
Lopes, H., Mendes, P. M. and Dinis, H.
Millimeter-Wave Systems for Real-Time Intraoperative Brain Tumor Resection Assistance.
DOI: 10.5220/0013399300003911
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 18th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2025) - Volume 1, pages 1085-1091
ISBN: 978-989-758-731-3; ISSN: 2184-4305
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
1085

real-time intraoperative information still needs more
development, especially in tumors where the main
treatment is the maximal removal of the mass. There
are several techniques and tools to help
neurosurgeons remove tumors, however, they come
with some drawbacks and limitations.
Tools like the exoscope, which is based on
positioning a camera alongside the surgeon, offering
two to three-dimensional high-resolution imaging on
a display monitor placed in front of the surgeon,
presents a learning curve for surgeons accustomed to
traditional microscopes, leading, in some cases, to the
change to the conventional operating microscope
during the surgery and has some substantial costs
(acquisition and maintenance), which may not be
feasible for all healthcare settings (Ariffin et al.,
2019; Montemurro et al., 2021).
The Fluorescence-guided surgery technique
provides real-time intraoperative tumor visualization
by using selective fluorescence compounds in tumor
cells to delineate cancer tissue during surgery
(Hadjipanayis et al., 2015). Nevertheless, it can still
cause some interpretation errors, is not available for all
types of brain cancer, and also necessitates the use of
specialized surgical visualization systems, resulting in
more costs for the hospital (Su et al., 2014).
Raman spectroscopy works by directing a single-
wavelength light beam onto a sample and observing
the scattered light as it interacts with the molecules
within the sample. However, this technique has a
weak intensity signal and long data acquisition and
processing times (Rivera et al., 2024).
The use of confocal microscopy, a technique that
creates a point source of light and eliminates out-of-
focus light, enabling high-resolution imaging deep
into tissues and optical sectioning for 3D
reconstructions, may blur and overlap regions of
hypercellularity, reducing confidence in the
classification (Elliott, 2020).
The more conventional intraoperative imaging
approaches, such as intraoperative magnetic
resonance imaging (iMRI) and intraoperative
computed tomography (iCT), have lengthy image
acquisition times and require the interruption of the
surgery, which translates into longer surgeries and
more time under anesthesia for the patient.
Additionally, these techniques are quite expensive
and are not available in most healthcare facilities.
The intraoperative ultrasound (iUS) normally
does not have enough spatial resolution for the
detection of microstructures or cellular elements.
With this, it is clear that there is a need for a way
to differentiate tissue during brain tumor resection
surgery that works in real-time, in situ and is low-
cost. Radiofrequency (RF) technology has shown an
increasing potential in the medical and healthcare
field because tumors and normal tissues have
different dielectric properties due to their different
tissue structure and vascularization, and generally,
cancer cells have a higher water content (Wang et al.,
2024). The RF short wavelengths may allow for the
achievement of higher spatial resolution, making
them very effective for sensing pathological changes.
Over the years, significant progress has been
made in characterizing the dielectric properties of
biological tissues, particularly up to 20 GHz.
However, studies on the dielectric properties of
human tumor tissues remain limited. This scarcity is
largely due to challenges in conducting
measurements, including the complex logistics of
systematic sample collection, proper handling, and
timely testing within hospital environments. These
practical constraints have hindered the
comprehensive study of tumor tissue dielectric
characterization. Additionally, the dielectric
properties of intracranial tumors appear to be
depending on histological class and malignancy
grade, showing significant intratumoral heterogeneity
(Kordić & Šarolić, 2023).
Nevertheless, Table 1 presents some of the results
reported in the literature, with the values calculated
separately for the real (𝛆
𝒓
′
) and imaginary (𝛆
𝒓
′′
) parts of
the measured permittivity as a percentage difference,
offering a concise overview of the dielectric
properties of tumor tissues.
Table 1: The average discrepancy in permittivity between
tumor tissues and their surrounding tissues is provided,
along with the temperature and frequency ranges reported
in the studies.
Authors
Tumor Tissue
∆𝛆
𝒓
′
(%)
∆𝛆
𝒓
′′
(%)
(Lu et al., 1992)
Glioma in comparison to white
matter
(0.005–0.5 GHz)
30
30
(Kordić &
Šarolić, 2023)
Meningioma in comparison to
white matter tissue
(0.5–18 GHz)
76.7
157.6
Meningioma in comparison to
gray matter tissue
(0.5–18 GHz)
11.6
16.7
To obtain values for dielectric properties above 20
GHz, empirical models can be employed, such as the
one proposed by Schepps and K. Foster (Schepps &
Foster, 1980). Figure 1 shows the dielectric properties
for both healthy brain tissue and brain cancer tissue,
providing a comparative analysis at higher
frequencies.
EM4Health 2025 - Special Session on Electromagnetic waves for healthcare
1086
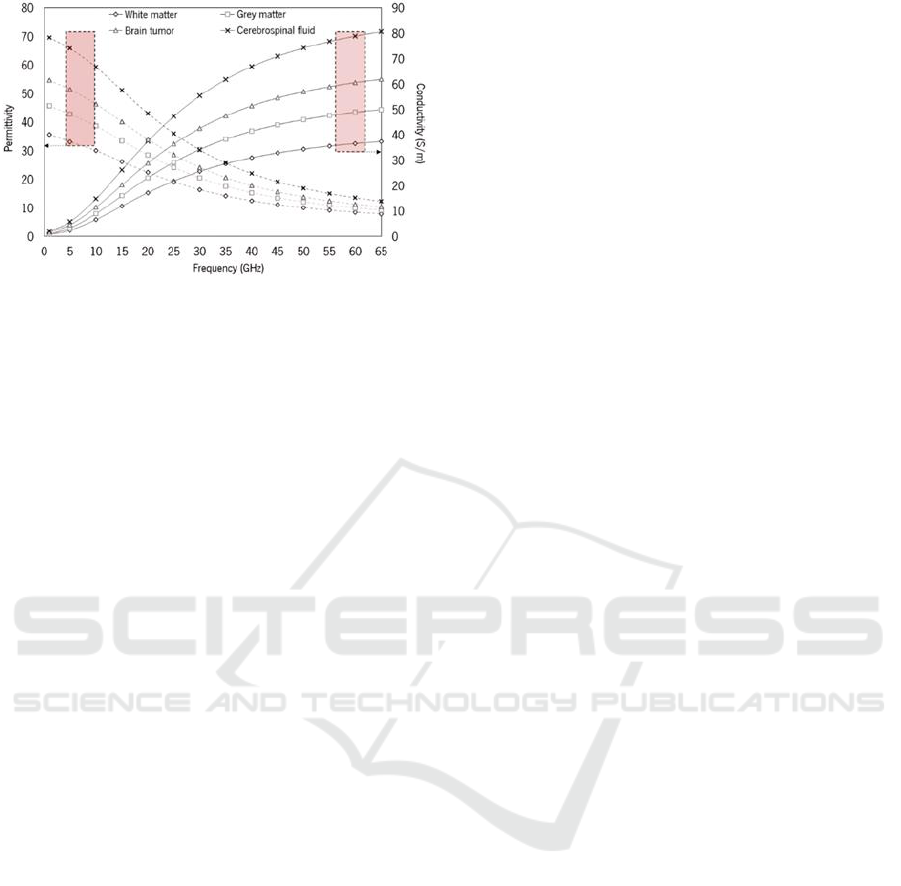
Figure 1: Representation of the variation in dielectric
properties, including conductivity and permittivity,
calculated for the various constituents of the brain and brain
tumor as a function of frequency (Cardoso, 2019).
2 RF-BASED DETECTION OF
TISSUES: POTENTIAL AND
CHALLENGES
Microwaves represent a form of electromagnetic
radiation characterized by frequencies that vary from
3 GHz to 30 GHz. Various electromagnetic
measurement methods and imaging techniques have
been utilized on biological tissues since the different
dielectric properties between healthy tissues and
cancerous tumor tissues have raised optimism about
employing microwaves for medical diagnostic
purposes.
With this, several studies and works have been
conducted on diagnostic tools, both with and without
contact, as well as imaging techniques using
microwaves (Çalışkan et al., 2015; Raihan et al.,
2017; Zhang et al., 2013). Most of these tools were
developed to detect specific healthy or cancerous
tissues, such as tumors. The main concept behind
using microwaves for diagnostics is the use of
transmitting antennas to emit electromagnetic waves
and receiving antennas to capture the scattered waves,
enabling the detection or distinction of certain tissues
(Çalışkan et al., 2015). Since different tissues absorb
varying amounts of energy due to their differing
dielectric properties, such as electrical conductivity
(σ) and relative permittivity (ε
𝑟
), these properties play
a crucial role in identifying different tissue types.
The primary advantages of using microwaves in
diagnostic tools include the harmless nature of this
type of radiation when used at low levels and its
relatively low cost, even for more complex systems,
when compared to iCT or iMRI. Additionally, the
availability of a relatively wide frequency range is a
significant benefit of using these techniques (Rosen
et al., 2002). However, despite the fact that RF waves
do not possess the resolution necessary to visualize
microstructures or surpass the resolution of iUS, the
latter uses systems of 4 to 10 MHz approximately,
which translates into larger probes, difficult to insert
in the small craniotomy hole. This can be overturned
by the use of higher frequencies, since it is possible
to develop antennas with small physical dimensions
allowing for smaller devices.
In 2019, (Alqadami et al., 2019) proposed a
“Wearable Electromagnetic Head Imaging System
utilizing a Flexible Wideband Antenna Array Based
on Polymer Technology for Brain Stroke Diagnosis”.
The system features eight high-efficiency, flexible,
lightweight, and robust antennas, designed
specifically for stroke applications. Scanning and
image reconstruction are completed in just 8 seconds.
The antennas offer a 54% fractional bandwidth (1.16-
1.94 GHz) and over 80% radiation efficiency,
satisfactory wave penetration in head tissues.
(Chowdhury et al., 2017) designed a wearable
pentagon-shaped antenna for brain tumor detection in
a compact form to be placed on the patient's head. It
was specifically developed to detect tumors at an
early stage. The antenna achieved satisfactory results,
demonstrating a frequency shift of 18 MHz in the
resonance frequency between a normal head and a
head with a tumor. The bandwidth of the proposed
antenna was 2.4-2.4835 GHz.
(Mohammed et al., 2014) suggested a microwave
imaging system capable of producing brain images to
identify the position and extent of brain injuries, such
as strokes, with a bandwidth of 3 GHz (1-4 GHz). The
system features a semi-elliptical array of 16/32
antenna elements mounted on an adjustable platform,
a data acquisition unit, a Vector Network Analyzer
(VNA), and a computer. It offers portability and
executes scans in 20 seconds. Specifically designed
for stroke detection, the system accurately identifies
the presence of a stroke and predicts its location
within a margin of a few millimeters.
A common problem of the systems previously
described is their size. None of the systems are
adequate for use during surgery, especially
considering the small opening created by the surgeons
to access the brain tumor for resection.
A way to address this issue is the use of higher
frequencies, such as millimeter waves (mmWave).
Because of the small wavelength, mmWave devices
facilitate large antenna arrays packed in miniature
physical dimensions, allowing for packing more
antenna elements at mmWave frequencies than at
microwave frequencies, resulting in a narrower beam
Millimeter-Wave Systems for Real-Time Intraoperative Brain Tumor Resection Assistance
1087

and increased resolution (Chittimoju & Yalavarthi,
2021).
The short wavelengths of mmWave allow for the
achievement of higher spatial resolutions at the cost
of reduced penetration depths (600 μm to 1.2mm into
the body), making them very effective for sensing
pathological changes in different skin layers or the
outer tissue layers of excised organs (Mirbeik-
Sabzevari et al., 2018). Furthermore, existing
mmWave technologies have demonstrated a
significant decrease in unnecessary biopsies,
indicating a potential pathway for continued research
on non-invasive early cancer screening in the future.
Such technologies and systems are going to be shown
in the next section.
3 APPLICATIONS OF mmWAVE
TECHNOLOGY IN MEDICAL
DIAGNOSIS AND TREATMENT
Over the years, there have been developed some
prototypes of systems and tools that use mmWave for
medical diagnostics and has been studied the potential
therapy effects of these waves. In this section, some
of these prototypes will be shown, as well as the setup
used and the potential limitations of each.
(Töpfer et al., 2015) proposed a miniaturized
broadband mmWave near-field probe with a conical
probe tip, designed to operate at frequencies between
90 and 104 GHz, for skin cancer identification. The
probe utilizes a dielectric waveguide with a high-
resistivity silicon core, with the sensing end tapered
into a conical tip. This tapering focuses the electric
field into a small area, enhancing the probe’s lateral
resolution. Since the dielectric properties of the skin
vary between individuals and across different body
locations, the probe performs differential
measurements, i.e., the signal from a suspicious area
is compared with the surrounding tissue, enabling the
probe to detect subtle differences that may indicate
the presence of cancer. For the s-parameter measure,
the waveguide was connected to a VNA with
mmWave extension heads to 110 GHz through a
coaxial adapter. The network analyzer was calibrated
by thru-reflect-line (TRL) or one-port calibration
using WR-10 waveguide standards at the coax-to-
waveguide adapter output plane.
The probe demonstrated high responsivity at 96
GHz, with an S11 change of 1.83 dB for a tip size of
0.6 mm × 0.5 mm, and a sensing depth of 0.3–0.4 mm.
The long-term measurement stability was 0.66% over
8 hours, ensuring consistent and reliable
performance.
Still in skin cancer detection, a novel multi-tone
mmWave radar sensor was presented (Arab et al.,
2020). It is based on a low-cost Miniature Hybrid
Microwave Integrated Circuit design (MHMIC) at 77
GHz. The proposed radar system uses a six-port
interferometer with I/Q demodulation to recover
information. It employs a linear passive mmWave
circuit with four 90° hybrid couplers and a phase
shifter, fabricated on a ceramic substrate using
MHMIC technology. An 8×2 microstrip patch
antenna array is utilized for enhanced performance
and coverage. In the measurement setup, the source
frequency is set to 12.83 GHz with a 10 dBm power
output and the multiplier will generate a 77 GHz
signal with 0 dBm power at the input of the parallel
line coupler.
This sensor had promising results, showing that it
was able to distinguish between dry hand skin, wet
hand skin and water. However, these results are with
a limited number of samples, needing more to
represent the diversity of the human skin better.
Additionally, the calibration steps are not mentioned,
which are crucial for an accurate measure.
(Mansutti et al., 2020) proposed and designed a
probe for early skin cancer detection. The
measurement setup consists of connecting the probe
to the VNA via a high-frequency cable and mounted
on a Computer Numerical Control machine, used to
determine the correct height of the probe for
measurements, ensuring direct contact with the
surface material under test. The measurement
procedure involves scanning the probe over a 2D grid
with a 1 mm step size in both directions, acquiring
data from multiple points on the skin model since an
imaging algorithm is intended to be applied, to
generate tissue structure maps. The resonance
frequency obtained was nearly 40 GHz and a lateral
sensitivity and detection depth of 0.2 mm and 0.55
mm respectively.
The mmWave technology is also used for the
development of imaging systems (Chao et al., 2012).
The article proposes a method for breast cancer
imaging using quasi-optical free space mmWave
spectroscopy, generating 2D and 3D tissue structure
maps to differentiate normal tissue from cancerous
tissue. The measurement setup employs a quasi-
optical free space mmWave spectrometer with
tunable backward wave oscillators operating in the
30-120 GHz range. Key components include an
isolator, modulator, horn antennas, focusing lenses,
and a Schottky diode detector. Despite the proposed
system being capable of the referred 2D and 3D tissue
EM4Health 2025 - Special Session on Electromagnetic waves for healthcare
1088

structure maps, the relatively large diameter of the
energy beam used in the measurement limits the
spatial resolution of the image and the low power of
the mmWave used limits the depth penetration.
Still in the use of mmWave for imaging systems,
(Mirbeik et al., 2022) developed a high-resolution
mmWave imaging system for skin cancer detection.
The system comprises a set of antennas designed to
transmit and receive mmWave within a specific
frequency band optimized for skin imaging. These
antennas, using an ultra-wide synthetic bandwidth of
98 GHz (12-110 GHz), achieved by integrating two
sub-bands, scan the target skin area by emitting
mmWave and capture the reflected signals. The
antipodal Vivaldi antennas ensure perfect impedance
matching and stable gain across the frequency range,
enhancing the system's performance during scanning.
To further improve precision, the system incorporates
a motorized XYZ linear arm, enabling 3D scanning
of the target area. The data collected is processed
using a reconstruction algorithm that leverages the
dielectric properties of various tissue types to
generate detailed images, highlighting cancerous
tissue areas with high accuracy. Additionally, the
system operates in real-time, completing each
measurement in approximately 20 seconds, which
minimizes patient discomfort and mitigates the
effects of movement during the procedure. However,
despite the good results obtained, the system's
performance was not consistent across all skin lesion
classes.
4 PROPOSED mmWAVE
SYSTEM
Based on prior knowledge and advancements in
mmWave technologies, a system is proposed (Figure
2) to differentiate biological tissues based on the
dielectric properties. The system operates with
mmWave technology and is structured into several
key components, such as a signal generator (Agilent
83623B), a frequency multiplier (HAFMV4-187), a
mixer (QMC-MXB15-NBMCA), and a custom probe
developed in-house (Cardoso, 2019). The signal
generator produces an initial signal that is
upconverted to higher frequencies through a
multiplier. The signal generator produces a 13 GHz
continuous wave that is converted to 52 GHz through
the 4x multiplier, which is used as the local oscillator
(LO) of the mixer. The signal from the VNA
(Keysight E5071C), with a proposed frequency of 2
GHz, is the intermediate frequency (IF) and it will be
upconverted in the mixer, resulting in a 54 GHz
signal. After filtering (SWF-50346340-15-H1), this
signal is transmitted from the probe to interact with
the material under test (MUT).
Figure 2: Proposed mmWave system setup. Red lines
represent microwave signals, while green lines represent
mmWave signals.
The reflected signals are measured and analyzed
using the VNA, which calculates the S-parameters.
These parameters provide insights into the dielectric
characteristics of the MUT, enabling real-time
detection of changes in the tissue.
The proposed system's architecture allows for
modular testing and optimization of the components,
ensuring flexibility for adjustments in the frequency
range, signal power, or filtering capabilities.
Additionally, the combination of high-frequency
operation with precision measurement tools should
ensure reliable and repeatable results, crucial for
intraoperative applications.
The primary objective of this system is to test if it
is possible to adapt the existing equipment and a VNA
to detect subtle variations in tissue properties, such as
those between healthy and abnormal brain tissue. To
do this, human body phantoms with different
dielectric properties will be made, and it will be
attempted to distinguish them with the proposed setup
by analyzing the signal reflected by the phantom, in
order to validate and obtain the system sensibility, i.e,
the minimum detectable change in dielectric
properties that allows the differentiation between
healthy and tumor tissues. The human body phantoms
will be created with mixtures of deionized water,
Triton X-100 and diethylene glycol butyl ether
(DGBE), an alcohol, to test the system, as these are
standard ingredients for human body phantom
development (FCC, 1997).
It is expected that this system will pave the way
for the development of a stand-alone mmWave brain
tumor detector, as it will serve as an adaptable
platform to test different components and system
architectures.
Millimeter-Wave Systems for Real-Time Intraoperative Brain Tumor Resection Assistance
1089

5 CONCLUSIONS
This study highlights the potential of mmWave
technology for intraoperative applications,
particularly in distinguishing brain tissues during
oncological procedures. By leveraging the different
dielectric properties of tissues, mmWave systems
have demonstrated a capacity to provide accurate and
real-time feedback that can significantly assist
surgeons in differentiating between healthy and
tumor-affected regions. This approach addresses the
limitations of current imaging technologies, such as
low resolution or time delays, and offers a precise tool
for surgical guidance.
The use of mmWave frequencies is particularly
advantageous because the small wavelength enables
compact component design and high spatial
resolution, which are critical for detecting subtle
variations in tissue properties in a confined surgical
environment.
Based on the findings of the literature review, we
propose a system that, employing a VNA, aims to
study the possibility of detecting subtle variations in
tissue properties, in order to obtain the minimum
detectable change that allows the differentiation of
tissues. The next steps will focus on developing a
fully functional mmWave system for intraoperative
applications. This system will then be tested in
experimental settings to validate its performance,
accuracy, and reliability in differentiating between
phantoms with different dielectric properties.
Future work will include upgrades to improve the
probe's sensitivity and resolution, ensuring its
reliability for real-world intraoperative applications.
These advancements aim to further optimize the
system, making it a valuable tool for enhancing
surgical precision and improving patient outcomes.
Additionally, while the proposed system relies on a
VNA for signal measurement and analysis, its size,
complexity, and cost can pose challenges for practical
intraoperative applications. To address this,
alternative detection circuits, such as compact
spectrum analyzers or custom-designed integrated
circuits, could be explored. These options offer
potential for miniaturization and cost reduction while
maintaining adequate performance for detecting
variations in dielectric properties. Moreover, a stand-
alone system specifically designed to meet the
systems requirements is planned for development,
ensuring a more practical and efficient solution for
intraoperative use.
This initial study will open the way for precise
real-time intraoperative measurements and
potentially enhance surgical outcomes through real-
time differentiation of tissue types resorting to
mmWave technology.
REFERENCES
Alqadami, A. S. M., Bialkowski, K. S., Mobashsher, A. T.,
& Abbosh, A. M. (2019). Wearable Electromagnetic
Head Imaging System Using Flexible Wideband
Antenna Array Based on Polymer Technology for Brain
Stroke Diagnosis. IEEE Transactions on Biomedical
Circuits and Systems, 13(1), 124–134. IEEE
Transactions on Biomedical Circuits and Systems.
https://doi.org/10.1109/TBCAS.2018.2878057
Arab, H., Chioukh, L., Dashti Ardakani, M., Dufour, S., &
Tatu, S. O. (2020). Early-Stage Detection of Melanoma
Skin Cancer Using Contactless Millimeter-Wave
Sensors. IEEE Sensors Journal, 20(13), 7310–7317.
IEEE Sensors Journal.
https://doi.org/10.1109/JSEN.2020.2969414
Ariffin, M. H. M., Ibrahim, K., Baharudin, A., & Tamil, A.
M. (2019). Early Experience, Setup, Learning Curve,
Benefits, and Complications Associated with Exoscope
and Three-Dimensional 4K Hybrid Digital
Visualizations in Minimally Invasive Spine Surgery.
Asian Spine Journal, 14(1), 59–65.
https://doi.org/10.31616/asj.2019.0075
Brain and Other Nervous System Cancer—Cancer Stat
Facts. (2020).
https://seer.cancer.gov/statfacts/html/brain.html
Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R. L.,
Soerjomataram, I., & Jemal, A. (2024). Global cancer
statistics 2022: GLOBOCAN estimates of incidence
and mortality worldwide for 36 cancers in 185
countries. CA: A Cancer Journal for Clinicians, 74(3),
229–263. https://doi.org/10.3322/caac.21834
Çalışkan, R., Gültekin, S. S., Uzer, D., & Dündar, Ö.
(2015). A Microstrip Patch Antenna Design for Breast
Cancer Detection. Procedia - Social and Behavioral
Sciences, 195, 2905–2911.
https://doi.org/10.1016/j.sbspro.2015.06.418
Cardoso, V. C. D. S. (2019). Desenvolvimento de sonda
cirúrgica de ondas milimétricas para assistência na
ressecção de tumores neuronais [masterThesis].
https://repositorium.sdum.uminho.pt/handle/1822/775
08
Chao, L., Afsar, M. N., & Korolev, K. A. (2012). Millimeter
wave dielectric spectroscopy and breast cancer
imaging. 2012 7th European Microwave Integrated
Circuit Conference, 572–575.
https://ieeexplore.ieee.org/document/6483864
Chittimoju, G., & Yalavarthi, U. D. (2021). A
Comprehensive Review on Millimeter Waves
Applications and Antennas. Journal of Physics:
Conference Series, 1804(1), 012205.
https://doi.org/10.1088/1742-6596/1804/1/012205
Chowdhury, T., Farhin, R., Hassan, R. R., Bhuiyan, M. S.
A., & Raihan, R. (2017). Design of a patch antenna
operating at ISM band for brain tumor detection. 2017
EM4Health 2025 - Special Session on Electromagnetic waves for healthcare
1090

4th International Conference on Advances in Electrical
Engineering (ICAEE), 94–98.
https://doi.org/10.1109/ICAEE.2017.8255334
Delaidelli, A., & Moiraghi, A. (2024). Recent Advances in
the Diagnosis and Treatment of Brain Tumors. Brain
Sciences, 14(3), Article 3.
https://doi.org/10.3390/brainsci14030224
Elliott, A. D. (2020). Confocal Microscopy: Principles and
Modern Practices. Current Protocols in Cytometry,
92(1), e68. https://doi.org/10.1002/cpcy.68
FCC. (1997). OET bulletin 65: Evaluating compliance with
FCC guidelines for human exposure to radiofrequency
electromagnetic fields. 65.
Hadjipanayis, C. G., Widhalm, G., & Stummer, W. (2015).
What is the Surgical Benefit of Utilizing 5-
Aminolevulinic Acid for Fluorescence-Guided Surgery
of Malignant Gliomas? Neurosurgery, 77(5), 663.
https://doi.org/10.1227/NEU.0000000000000929
Kordić, A., & Šarolić, A. (2023). Dielectric Spectroscopy
Shows a Permittivity Contrast between Meningioma
Tissue and Brain White and Gray Matter—A Potential
Physical Biomarker for Meningioma Discrimination.
Cancers, 15(16), Article 16.
https://doi.org/10.3390/cancers15164153
Lu, Y., Li, B., Xu, J., & Yu, J. (1992). Dielectric properties
of human glioma and surrounding tissue. International
Journal of Hyperthermia: The Official Journal of
European Society for Hyperthermic Oncology, North
American Hyperthermia Group, 8(6), 755–760.
https://doi.org/10.3109/02656739209005023
Mansutti, G., Mobashsher, A. T., Bialkowski, K.,
Mohammed, B., & Abbosh, A. (2020). Millimeter-
Wave Substrate Integrated Waveguide Probe for Skin
Cancer Detection. IEEE Transactions on Biomedical
Engineering, 67(9), 2462–2472. IEEE Transactions on
Biomedical Engineering.
https://doi.org/10.1109/TBME.2019.2963104
Mirbeik, A., Ashinoff, R., Jong, T., Aued, A., &
Tavassolian, N. (2022). Real-time high-resolution
millimeter-wave imaging for in-vivo skin cancer
diagnosis. Scientific Reports, 12(1).
https://doi.org/10.1038/s41598-022-09047-6
Mirbeik-Sabzevari, A., Ashinoff, R., & Tavassolian, N.
(2018). Ultra-Wideband Millimeter-Wave Dielectric
Characteristics of Freshly Excised Normal and
Malignant Human Skin Tissues. IEEE Transactions on
Biomedical Engineering, 65(6), 1320–1329. IEEE
Transactions on Biomedical Engineering.
https://doi.org/10.1109/TBME.2017.2749371
Mohammed, B. J., Abbosh, A. M., Mustafa, S., & Ireland,
D. (2014). Microwave System for Head Imaging. IEEE
Transactions on Instrumentation and Measurement,
63(1), 117–123. IEEE Transactions on Instrumentation
and Measurement.
https://doi.org/10.1109/TIM.2013.2277562
Montemurro, N., Scerrati, A., Ricciardi, L., & Trevisi, G.
(2021). The Exoscope in Neurosurgery: An Overview
of the Current Literature of Intraoperative Use in Brain
and Spine Surgery. Journal of Clinical Medicine, 11(1),
223. https://doi.org/10.3390/jcm11010223
Petrecca, K., Guiot, M.-C., Panet-Raymond, V., &
Souhami, L. (2013). Failure pattern following complete
resection plus radiotherapy and temozolomide is at the
resection margin in patients with glioblastoma. Journal
of Neuro-Oncology, 111(1), 19–23.
https://doi.org/10.1007/s11060-012-0983-4
Raihan, R., Bhuiyan, M. S. A., Hasan, R. R., Chowdhury,
T., & Farhin, R. (2017). A wearable microstrip patch
antenna for detecting brain cancer. 2017 IEEE 2nd
International Conference on Signal and Image
Processing (ICSIP), 432–436.
https://doi.org/10.1109/SIPROCESS.2017.8124578
Rivera, D., Young, T., Rao, A., Zhang, J. Y., Brown, C.,
Huo, L., Williams, T., Rodriguez, B., & Schupper, A. J.
(2024). Current Applications of Raman Spectroscopy in
Intraoperative Neurosurgery. Biomedicines, 12(10),
2363. https://doi.org/10.3390/biomedicines12102363
Rosen, A., Stuchly, M. A., & Vorst, A. V. (2002).
Applications of RF/microwaves in medicine. IEEE
Transactions on Microwave Theory and Techniques,
50(3), 963.
Schepps, J. L., & Foster, K. R. (1980). The UHF and
microwave dielectric properties of normal and tumour
tissues: Variation in dielectric properties with tissue
water content. Physics in Medicine & Biology, 25(6),
1149. https://doi.org/10.1088/0031-9155/25/6/012
Šteňo, A., Buvala, J., Babková, V., Kiss, A., Toma, D., &
Lysak, A. (2021). Current Limitations of Intraoperative
Ultrasound in Brain Tumor Surgery. Frontiers in
Oncology, 11, 659048.
https://doi.org/10.3389/fonc.2021.659048
Su, X., Huang, Q.-F., Chen, H.-L., & Chen, J. (2014).
Fluorescence-guided resection of high-grade gliomas:
A systematic review and meta-analysis. Photodiagnosis
and Photodynamic Therapy, 11(4), 451–458.
https://doi.org/10.1016/j.pdpdt.2014.08.001
Surgery for brain tumours—Cancer Research UK. (2023,
March 31). https://www.cancerresearchuk.org/about-
cancer/brain-tumours/treatment/surgery/remove-brain-
tumour
Töpfer, F., Dudorov, S., & Oberhammer, J. (2015).
Millimeter-Wave Near-Field Probe Designed for High-
Resolution Skin Cancer Diagnosis. IEEE Transactions
on Microwave Theory and Techniques, 63(6), 2050–
2059. IEEE Transactions on Microwave Theory and
Techniques.
https://doi.org/10.1109/TMTT.2015.2428243
Wang, H., Lu, L., Liu, P., Zhang, J., Liu, S., Xie, Y., Huo,
T., Zhou, H., Xue, M., Fang, Y., Yang, J., & Ye, Z.
(2024). Millimeter waves in medical applications:
Status and prospects. Intelligent Medicine, 4(1), 16–21.
https://doi.org/10.1016/j.imed.2023.07.002
Zhang, H., Arslan, T., & Flynn, B. (2013). A single antenna
based microwave system for breast cancer detection:
Experimental results. 2013 Loughborough Antennas &
Propagation Conference (LAPC), 477–481.
https://doi.org/10.1109/LAPC.2013.6711945
Millimeter-Wave Systems for Real-Time Intraoperative Brain Tumor Resection Assistance
1091
