
Exploring Endothelial Cell Adhesion to High-Resolution 3D Printing
Materials for Advanced Organ-on-Chip Fabrication
Steffen Winkler
1 a
, Xenia Kraus
1 b
, Jasmin Huber
1
and Janina Bahnemann
1,2 c
1
Institute of Physics, University of Augsburg, 86159, Augsburg, Germany
2
Center for Advanced Analytics and Pre Augsburg, 86159, Augsburg, Germany
janina.bahnemann@uni-a.de
Keywords: Biocompatibility, 3D Printing, Cell Adhesion, Endothelial Cells, Organ-on-Chip.
Abstract: With advancements in resolution, 3D printing is emerging as a transformative technology for the rapid fabri-
cation of cell culture systems, including organ-on-chip platforms. For successful integration into cell culture
environments, 3D printing materials must not only exhibit general biocompatibility but also support direct
cell adhesion for on-chip cultivation. In this study, we investigated the adhesion of human umbilical vein
endothelial cells (HUVECs) to two 3D printing materials, AR-M2 and M2S-HT90, under varying sterilization
conditions involving heat steam sterilization and ethanol disinfection. Our findings reveal that specific com-
binations of these sterilization techniques significantly enhance cell adhesion, achieving levels comparable to
standard cell culture plates. However, alterations in the 3D printing mode resulted in a complete loss of cell
adhesion, underscoring the critical impact of printing parameters on the material surface properties.
1 INTRODUCTION
Organ-on-chip (OOC) systems simulate increasingly
complex tissue and organ functions and serve as
novel cell culture platforms – enabling both a deeper
understanding of (patho)physiological processes in
academia and industrial applications such as drug dis-
covery. They are increasingly recognized for their es-
sential role in mimicking the complexity of tissues
and organs, thereby enhancing the physiological rel-
evance of experimental results (Leung et al., 2022).
Traditionally, OOCs are microfluidic systems that
consist of microchannels and chambers enabling ac-
tive perfusion of the culture with medium. Fabrica-
tion of microfluidic systems remains a considerable
challenge. Traditional manufacturing techniques are
time- and cost-intensive such as soft lithography, mi-
cro-milling, injection molding, and etching, which
necessitate the use of highly specialized equipment
and cleanroom facilities.
Recent advancements in 3D printing have intro-
duced promising alternatives for fabricating OOCs
more cost-effectively and with reduced developmen-
tal timelines (Meyer et al., 2023; Siller et al., 2020).
a
https://orcid.org/0000-0001-7386-9477
b
https://orcid.org/0000-0002-1186-5443
c
https://orcid.org/0000-0002-7008-1673
Nonetheless, there are only few peer-reviewed stud-
ies evaluating the biocompatibility of 3D-printed ma-
terials that can be printed in high-resolution (Siller et
al., 2019; Winkler et al., 2022). Biocompatibility as-
sessments are typically conducted according to estab-
lished guidelines, such as the International Organiza-
tion for Standardization (ISO) norms (e.g., ISO
10993) and United States Pharmacopeia (USP) Class
VI standards.
Despite these assessments, the direct growth of
cells on 3D-printed materials – specifically the adhe-
sion of cells to the material surface – is rarely inves-
tigated, as this is not a mandatory criterion in ISO-
based biocompatibility evaluations. However, cell
adhesion of a given cell line, is crucial for the devel-
opment of OOCs. It facilitates the use of cell culture
systems that are printed in a single step for immediate
on-chip cell seeding. When investigating the adhesion
properties of a 3D printing material, material post-
processing, including the sterilization method, must
be considered, since it can significantly influence the
materials surface properties and the amount of poten-
tially toxic leachables.
Winkler, S., Kraus, X., Huber, J. and Bahnemann, J.
Exploring Endothelial Cell Adhesion to High-Resolution 3D Printing Materials for Advanced Organ-on-Chip Fabrication.
DOI: 10.5220/0013418600003911
In Proceedings of the 18th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2025) - Volume 1, pages 1063-1067
ISBN: 978-989-758-731-3; ISSN: 2184-4305
Copyright © 2025 by Paper published under CC license (CC BY-NC-ND 4.0)
1063
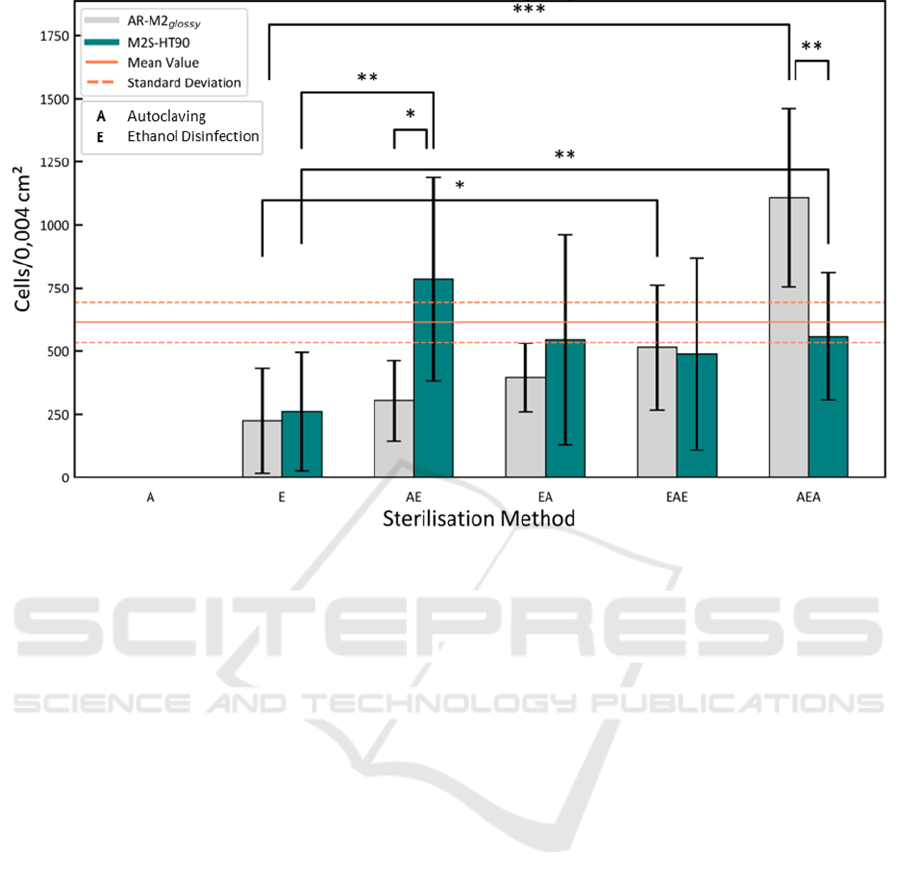
Figure 1: Cell adhesion of human umbilical vein endothelial cells (HUVEC) to material slides of the 3D printing materials
AR-M2 (printed in glossy mode) and M2S-HT90 for different sterilization/disinfection procedures using autoclaving (A)
and/or ethanol (E). Mean and standard deviation of the control is indicated by orange lines. Level of significance as indicated
by asterisks, *p < 0.05; **p < 0.01; ***p < 0.001.
In this study, two materials designed for high-res-
olution 3D printing of microfluidic cell culture sys-
tems were evaluated for their ability to support the
adhesion of human umbilical vein endothelial cells
(HUVEC). The results revealed that different combi-
nations of sterilization methods and printing modes
had a pronounced impact on cell adhesion. The find-
ings underscore the importance of material selection,
post-processing, and evaluation in advancing the de-
velopment of functional and reliable OOCs.
2 RESULTS AND DISCUSSION
Two 3D-printing materials, AR-M2 (printed with
AGILISTA-3200 W) and M2S-HT90 (printed with
ProJet MJP 2500 Plus), were tested for their adhesion
properties to HUVEC. Hence, the cells were culti-
vated on thin 48-well material slides (see Figure S1
in the Appendix), which were sterilized using differ-
ent combinations of heat steam sterilization (auto-
claving, (A)) and ethanol disinfection (E) as two of
the most popular sterilization/disinfection techniques.
Both materials are described by the manufacturers as
biocompatible according to ISO 10993 and/or USP
Class VI and can be printed in high resolution for the
fabrication of microfluidic structures and OOCs.
The cell confluence (as cell count per imaging
area of 0.004 cm²) after 24 h cultivation on AR-M2
(printed in glossy mode) and M2S-HT90 material
slides for different sterilization procedures is summa-
rized as presented in Figure 1. Noticeably, the conflu-
ence increases with an increased number of steriliza-
tion/disinfection steps. Slides that were only auto-
claved (A) facilitated no cell growth at all, while eth-
anol disinfection (E) with 225 ± 208 and 261 ± 234
cells/0.004 cm² shows a significantly impaired adhe-
sion compared to the control with 614 ± 78
cells/0.004 cm². For the M2S-HT90 material all com-
binations of ethanol and autoclaving treatments
showed no significant difference to a 48-well plate
and thus enable the use of the material for direct on-
chip cultivation. Considering these findings, it is hy-
pothesized that potential leachables are increasingly
released to the surface of the material due to the heat
of the autoclaving process and lead to a fully impaired
cell adhesion. In turn, additional extraction by ethanol
removes these leachables and a combination of both
sterilization methods leads to an overall decrease of
leachables inside the material and potential cytotoxi-
city. However, for the AR-M2
glossy
highest confluence
microOrganChip 2025 - Special Session on Organ on Chip Micro-Devices
1064

was found for the AEA sterilization sequence. Since
also ethanol can diffuse into the material, it may not
be removed by subsequent washing steps. The heat of
a second autoclaving step could reduce a possible
negative effect of ethanol and thus further reduces im-
pairments of cell attachment and growth. In addition,
representative images of the control and the best per-
forming sterilization/disinfection procedures are pre-
sented in Figure S2 showing a similar cell distribution
and morphology.
Importantly, the AR-M2 material was also printed
in in the matte printing mode, where in contrast to the
glossy printing mode additional support material co-
vers the top surface of the material slide. Surprisingly,
the change of the printing mode led to a complete loss
of cell adhesion under all tested conditions (data not
shown). This is explained by a complete change of the
surface structure in the matte printing mode, which
increases the surface roughness. The findings demon-
strate the importance of the printing mode and its dra-
matic influence on cell adhesion.
3 CONCLUSIONS
In this work, two materials AR-M2 and M2S-HT90 –
suitable for high resolution 3D printing of microflu-
idic and OOC systems – were investigated on their
adhesion properties to HUVEC using several differ-
ent sterilization procedures. The findings show that
combinations of the sterilization techniques enable
the use of the materials for direct cultivation of HU-
VEC with a comparable confluence to standard cell
culture plates after 24 h cultivation. Furthermore, it
revealed that solely sterilizing by autoclaving or dis-
infecting using ethanol is insufficient and is accom-
panied by an impaired cell attachment. Another key
finding is the strong effect of the printing mode. The
matte printing mode of the AGILISTA-3200 W re-
sults in a loss of cell adhesion at all tested con-
ditions. Since the surface structure in generally
highly dependent on a variety of printing pa-
rameters, change of these parameters can gen-
erally remove or restore cell adhesion of 3D
printing materials.
In contrast to few publications focussing
solely on the biocompatibility using extraction
media according to ISO-10993, we enabled the
use of the printing materials for direct cell
growth on the inner surface of future cell cul-
ture systems. It enables the development of
OOC systems that are fabricated as a single part
removing the need for the integration of exter-
nal materials and thus further reducing fabrica-
tion and development times.
4 EXPERIMENTAL SECTION
4.1 Fabrication and Post-Processing of
3D-Printed Parts
The 3D-printed parts were designed using Solid-
Works 2024 (Dassault Systems Deutschland GmbH,
Germany) and printed using two different high reso-
lution multi-jet 3D printers, the ProJet MJP 2500 Plus
(3D Systems, USA) for printing of the polyacrylate
VisiJet M2S-HT90 (3D Systems, USA) and the
AGILISTA-3200 W (Keyence, Germany) for
printing of the polyacrylate AR-M2 (Keyence,
Germany) material, which was printed in two
configurations, glossy and matte.
Post-processing of the VisiJet M2S-HT90 ma-
terial included removal of the support material Visi-
Jet® M2 Sup (3D Systems, USA) with the following
steps: (1) detachment of the parts from the print plat-
form after 10 min incubation at -20 °C, (2) incubation
in a heat steam bath for 45 min, (3) incubation in a
paraffin oil bath (15 min, 65 °C), (4) incubation in a
ultrasonic paraffin oil bath (15 min, 65 °C), (5) 3x in-
cubation in a ultrasonic bath with ddH
2
O and deter-
gent (15 min, 65 °C) and (6) incubation in an ultra-
sonic bath with ddH2O only (15 min, 65 °C).
Post-processing of the AR-M2 material was per-
formed similarly without step (1-3). The two possible
printing modes glossy or matte were selected by
changing a single setting in the printer software Mod-
elling Studio (Keyence, Germany).
The following methods of sterilization were se-
lected for the experiment: autoclaving at 121°C for 30
min, and treatment with 96% ethanol (VWR, USA) in
various combinations. Treatment with ethanol was
carried out in an ultrasonic bath at 35°C for 1 h, fol-
lowed by an evaporation phase. The printed parts
were then placed in Phosphate Buffered Saline (PBS,
Capricorn Scientific, Germany) at room temperature
for 1 h, and dried.
4.2 Cell Culture Experiments
HUVEC were cultured in EGM-2 (Endothelial cell
growth medium-2, PromoCell, C-22011) in a density
of 6000 cells/cm
2
at 37 °C in a controlled environ-
ment of 5 % CO
2
and ≥ 95 % humidity. Culture me-
dium was supplemented with 10% fetal calf serum
Exploring Endothelial Cell Adhesion to High-Resolution 3D Printing Materials for Advanced Organ-on-Chip Fabrication
1065

(FCS, Capricorn Scientific GmbH, Germany) and
0.5% Gentamycin (VWR, USA).
For biocompatibility testing, HUVEC were
seeded in a density of 4.5 ∙ 10
5
cells/cm
2
on a single
slide of the 3D printing material inside a 48-well plate
(Sarstedt, Germany) (2.86 ∙ 10
5
/well) to reach an es-
timated cell confluence of about 80 % after 24 h cul-
tivation. To fix the slides on the bottom of the plate a
3D-printed cylinder was plugged into the 48-well (s.
Figure S1 in the Appendix). Each condition was per-
formed in triplicate, with 48-well plates devoid of ma-
terial slides serving as the control.
After cultivation, the cells were fixed for 30 min
at RT with 4 % paraformaldehyde (PFA, VWR,
USA) diluted in phosphate buffered saline (PBS)
(Capricorn Scientific, Germany). Cell nuclei were
stained with Hoechst 33342 dye (1:1000, Thermo
Fisher Scientific, Germany). Actin filaments were
stained with Phalloidin iFluor 555 Reagent-Cyto-
Painter (abcam, GBR; diluted 1:1000 in PBS with 1%
BSA (Sigma Aldrich Chemie GmbH, Germany)).
Cells were imaged using a Keyence BZ-X800 flu-
orescence microscope (Keyence, Germany) with a
20x objective. The observed imaging area was
0.004 cm². Cells were counted on three pictures of
each triplicate.
4.3 Statistical Analysis
Each condition was performed in triplicates, with 48-
well plates devoid of material slides serving as the
control. Levels of significance were analyzed using
one-way analysis of variance (ANOVA). Differences
were considered as significant at p < 0.05. Signifi-
cance levels were indicated with *p < 0.05, **p < 0.01
and ***p < 0.001.
ACKNOWLEDGEMENTS
We acknowledge the support by the program “For-
schungspotentiale besser nutzen!” of the University
of Augsburg.
REFERENCES
leung, C. M., Haan, P. De, Ronaldson-Bouchard, K.,
Kim, G.‑A., Ko, J., Rho, H. S., Chen, Z., Habibo-
vic, P., Jeon, N. L., Takayama, S., Shuler, M. L., Vun-
jak-Novakovic, G., Frey, O., Verpoorte, E., &
Toh, Y.‑C. (2022). A Guide to The Organ-On-A-Chip.
Nature Reviews Methods Primers, 2(1).
https://doi.org/10.1038/s43586-022-00118-6
Meyer, K. V., Winkler, S., Lienig, P., Dräger, G., &
Bahnemann, J. (2023). 3d-Printed Microfluidic Perfu-
sion System for Parallel Monitoring of Hydrogel-Em-
bedded Cell Cultures. Cells, 12(14).
https://doi.org/10.3390/cells12141816
Siller, I. G., Enders, A., Steinwedel, T., Epping, N.‑M.,
Kirsch, M., Lavrentieva, A., Scheper, T., &
Bahnemann, J. (2019). Real-Time Live-Cell Imaging
Technology Enables High-Throughput Screening to
Verify in Vitro Biocompatibility of 3D Printed Materi-
als. Materials (Basel, Switzerland), 12(13).
https://doi.org/10.3390/ma12132125
Siller, I. G., Enders, A., Gellermann, P., Winkler, S.,
Lavrentieva, A., Scheper, T., & Bahnemann, J. (2020).
Characterization of a customized 3D-printed cell cul-
ture system using clear, translucent acrylate that ena-
bles optical online monitoring. Biomedical Materials
(Bristol, England), 15(5), 55007.
https://doi.org/10.1088/1748-605X/ab8e97
Winkler, S., Meyer, K. V., Heuer, C., Kortmann, C.,
Dehne, M., & Bahnemann, J. (2022). Invitro biocom-
patibility evaluation of a heat-resistant 3D printing ma-
terial for use in customized cell culture devices. Engi-
neering in Life Sciences, 22(11), 699–708.
https://doi.org/10.1002/elsc.202100104
APPENDIX
Figure S1: CAD of the 3D-printed material slide (bottom)
(9x1 mm) and cylinder (top) (9x15 mm) for the investiga-
tion of the endothelial cell adhesion.
microOrganChip 2025 - Special Session on Organ on Chip Micro-Devices
1066
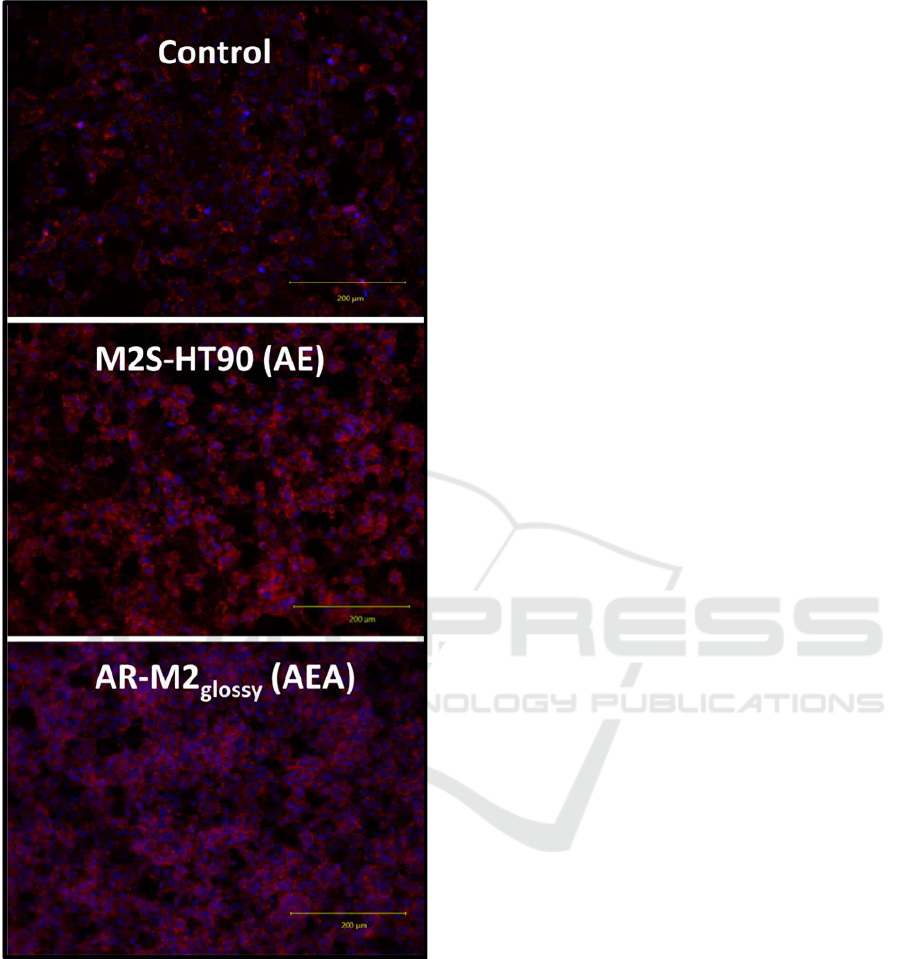
Figure S2: Representative images (20x objective) of phal-
loidin- and Hoechst 33342-stained HUVECs after 24 h cul-
tivation on 48-well plates as control and M2S-HT90 and
AR-M2
glossy
3D-printed material slides at the best perform-
ing sterilization/disinfection procedures AE and AEA (A:
autoclaving; E: ethanol disinfection).
Exploring Endothelial Cell Adhesion to High-Resolution 3D Printing Materials for Advanced Organ-on-Chip Fabrication
1067
