
Industry 4.0 Information Systems for Materials Circularity in Supply
Chains: Industry Issues and Research Directions
Soujanya Mantravadi
1a
and Brian Vejrum Wæhrens
2b
1
Department of Engineering, University of Cambridge, 17 Charles Babbage Road, Cambridge, U.K.
2
Department of Materials & Production, Aalborg University, Fibigestræde 16, Aalborg, Denmark
Keywords: Industry 4.0, Manufacturing Sustainability, Circular Economy, Manufacturing Operations Management
(MOM), Supply Chain Integration, Industry Policy, Traceability, Net-Zero, Knowledge Management.
Abstract: The purpose of this paper is to explore the role of information systems in manufacturing to support material
circularity practices in the supply chain. The paper attempts to study the usefulness of manufacturing
operations management (MOM) systems, particularly manufacturing execution systems (MES), in enabling
traceability and supply chain integration for tracking product material details. Theoretical propositions made
on MOM systems for materials circularity (based on the literature study) were empirically examined using
needs assessment from two case companies with complex product material requirements. Based on the
qualitative analysis of propositions and empirical findings, the paper identified traceability-enabled methods,
supply chain integration, and the adoption of Industry 4.0 technologies as potential enablers for achieving
materials circularity goals. As a result, the priorities for developing research agenda in this area to design
factories of the future and to achieve Industry 4.0 vision that supports circular economy were established.
Future research directions are put forward and future work will include an in-depth case study analysis to
explore the role of Industry 4.0-compliant MOM systems to meet evolving regulatory demands and
operational scalability across the supply chain.
1 INTRODUCTION
Materials Circularity in Manufacturing: One of
the biggest challenges manufacturing companies
worldwide currently face is adapting to evolving
sustainability compliance requirements while
tailoring their manufacturing operations and
equipment. For example, a global electronics
manufacturer may need to comply with stricter
regulations on carbon emissions and waste
management while simultaneously implementing
advanced automation and Industry 4.0 technologies.
This could involve retrofitting production lines with
energy-efficient equipment and materials recycling in
the factory.
The concept of material circularity covers a
much broader scope with implications for supply
chains, necessitating changes across all aspects of the
supply chain. It requires the adoption of modern
equipment, particularly advanced enterprise
a
https://orcid.org/0000-0001-9382-8314
b
https://orcid.org/0000-0001-6140-5587
information systems, to achieve sustainability goals.
However, this transition remains a work in progress
within the manufacturing industry and demands
research attention to address existing knowledge gaps
and industrial challenges.
The circular economy (CE), as highlighted by the
European Commission (EU) (European Union,
2015), calls for a lifecycle approach. However, its
core principle in practice focuses on increasing
material circularity by converting materials at the end
of their service life into resources for new
applications (European Union, 2015).
Materials circularity in manufacturing refers to
the concept of creating a closed-loop system for
materials, where resources are used efficiently, waste
is minimized, and materials are continually recycled
and reused in the production process. This approach
aims to reduce the environmental impact of
manufacturing activities by promoting sustainability
Mantravadi, S. and Wæhrens, B. V.
Industry 4.0 Information Systems for Materials Circularity in Supply Chains: Industry Issues and Research Directions.
DOI: 10.5220/0013482900003929
In Proceedings of the 27th International Conference on Enterprise Information Systems (ICEIS 2025) - Volume 2, pages 925-931
ISBN: 978-989-758-749-8; ISSN: 2184-4992
Copyright © 2025 by Paper published under CC license (CC BY-NC-ND 4.0)
925

and minimizing the depletion of natural resources
(Dumée, 2022).
Many manufacturing companies globally are
adjusting their manufacturing systems and practices
to meet future regulations of circular economy. Some
of the practices of materials circularity in
manufacturing include: Recycling and Reuse, Waste
reduction, Design for sustainability,
Remanufacturing, and Materials traceability.
EU and International Policies Associated With
Materials Circularity: several EU and international
policies are associated with the concept of circular
economy, which include materials circularity. Some
of these are https://commission.europa.eu/index_en:
• Circular Economy Action Plan (CEAP): The
CEAP is an EU initiative that presents
strategies and actions to promote a circular
economy. It covers product design, waste
management, recycling targets, and measures
to reduce the environmental impact of
products.
• Waste Framework Directive: It establishes the
legislative framework for waste management
in the EU. It sets out key principles, including
waste hierarchy, extended producer
responsibility, and recycling targets.
• Directive on single-use plastics: This directive
aims to reduce the impact of certain plastic
products on the environment, including bans
or restrictions on single-use plastic items and
promotion of circular design.
• Ecodesign Directive: This sets requirements
for the environmental performance of energy-
related products, promoting circularity by
encouraging longer product lifetimes, easier
repairability, and recyclability.
• Plastics Strategy: The EU Plastics Strategy
focuses on improving the economics and
quality of plastics recycling, reducing single-
use plastics, and promoting eco-friendly
alternatives.
Several international policies support the circular
economy with implications for manufacturing. The
United Nations (UN) Sustainable Development
Goals, particularly Responsible Consumption and
Production, emphasize sustainable practices. The
Basel Convention regulates hazardous waste
movements and management, while the Paris
Agreement indirectly promotes circular principles
through resource efficiency. The UN Environment
Programme advances initiatives on sustainable
consumption, and the OECD provides guidelines for
circular economy strategies for member countries.
Regulations and recommendations from various
governing bodies make it evident that stricter rules for
manufacturing are on the horizon, and manufacturers
must quickly adapt to these growing challenges.
However, many brownfield manufacturing
enterprises continue to rely on traditional
manufacturing methods and legacy equipment, which
hinder their ability to meet these evolving
requirements.
While many studies highlight the importance of
information systems in manufacturing supply chains
to achieve circular economy goals (Awan et al., 2021)
(Chhimwal et al., 2022), the roadmap for their
implementation remains unclear. Exisiting literature
highlights Industry 4.0 as an enabler of circular
supply chains (Gebhardt et al., 2022; Taddei et al.,
2022). (Zeiss et al., 2021) mention that product design
for reuse addresses repairability and upgradeability,
where digital processes and flexible platforms enable
design, analysis, and collaboration by facilitating
disassembly and reuse. They highlight Industry 4.0
technologies, such as the Internet of Things, Big Data
Analytics, and cloud computing, as beneficial for
circular supply chains.
Traditionally, enterprise information systems in
manufacturing were designed to enhance operational
efficiency, leaving their potential role in supporting
circular economy objectives underexplored. There
are limited studies in this area in manufacturing. This
demands further inquiry into the future operational
needs of factories and supply chains to use
information systems accordingly.
Motivated by this need, this paper examines how
manufacturing companies are adapting to these
regulations and identifies which factory information
systems have the potential to address these challenges
in supply chains—a question that has not been widely
studied in the existing literature.
Section 2 introduces the theoretical framing to the
research, Section 3 describes the approach, followed
by an analysis of company case study narratives in
Section 4. Section 5 concludes the position and
presents the future work.
2 MANUFACTURING
INFORMATION SYSTEMS FOR
MATERIALS CIRCULARITY
Enterprise information systems in manufacturing,
such as enterprise resource planning (ERP) and
ICEIS 2025 - 27th International Conference on Enterprise Information Systems
926
MES/MOM play a crucial role in promoting materials
circularity within the context of a circular economy.
These systems help manage and optimize the flow of
materials through various stages, from production to
consumption and recycling.
In Industry 4.0, these systems leverage
technologies such as Internet of Things (IoT) sensors
visibility and traceability of materials, which are
essential for supporting recycling, reuse, and
remanufacturing processes. ERP systems can
integrate data from various functions and are capable
of monitoring material usage. MES/MOM can
provide almost real-time product centric data for
decision-making on material recovery, reuse and
remanufacturing processes. Product Lifecycle
Management (PLM) systems can document the
environmental impact of materials and products,
helping manufacturers design for circularity.
A proven example of traceability is of RFID tags
in the automotive industry to track the recycling of
vehicle parts. By using such tracking capabilities,
companies can ensure that materials such as metals,
plastics, and composites are recovered and reused
rather than discarded.
Requirements of future factories: Given the
regulations outlined in Section 1, numerous
accountability and reporting issues arise. With the
changing regulations, manufacturers need to rethink
their computer-based information systems and use
advanced manufacturing operation management
systems to address these issues. A study (Omair et al.,
2024) suggested that manufacturing information
systems for materials circularity, in the context of
recycled plastic (RP), face challenges due to the high
variability in quality, composition, properties, and
lead time of RP. These variations create uncertainty
in material requirement planning, making it difficult
to optimize quantities and reorder times (Omair et al.,
2024).
Future factories will also require advanced
Internet of Things (IoT) capabilities to optimize
operations through real-time monitoring, tracking,
and control, enabling efficient use, extended product
lifecycles, and increased resource utilization for
circular economy (Uhrenholt et al., 2023). This is in
line with the Industry 4.0 vision of data-driven
decision-making, supported by predictive and
prescriptive analytics. MES/MOM, being a factory
database and a manufacturing cockpit is well
positioned to integrate with Industrial IoT to support
the transition towards material circularity in
manufacturing supply chains.
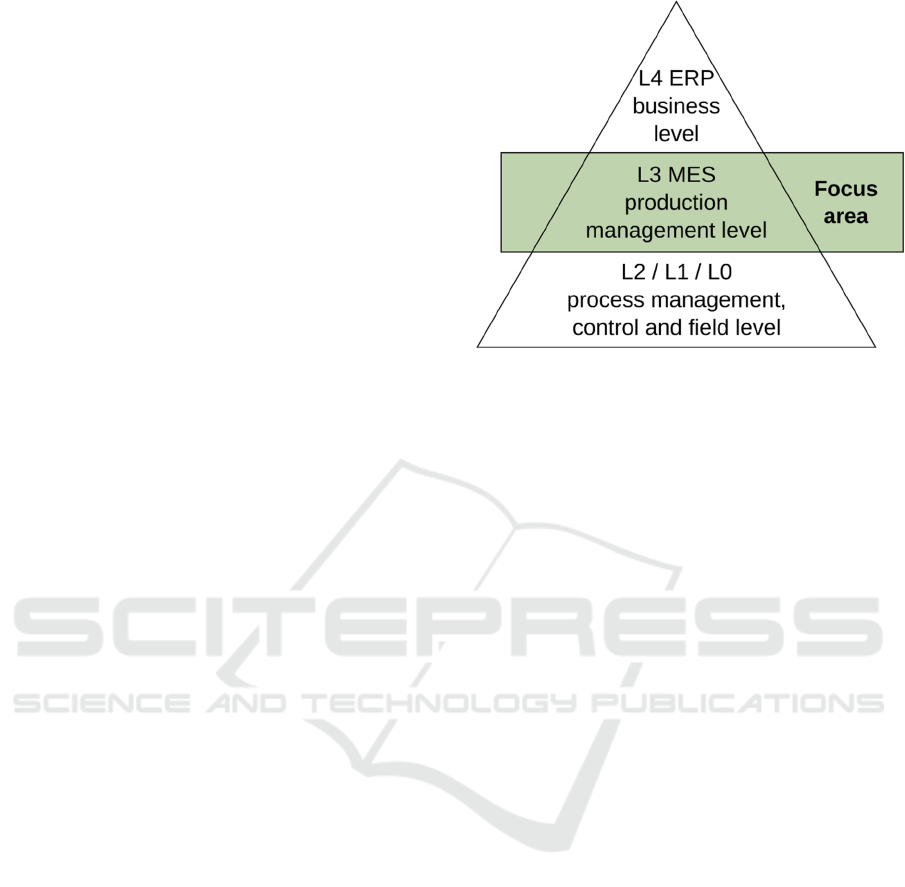
Figure 1 presents the MES/MOM position in the
manufacturing information systems.
Figure 1: ISA 95 Levels of functional hierarchy in a
manufacturing enterprise. (Scholten, 2007)
3 METHODOLOGY
We used a combination of selective literature review
and case study approach to explore the role of MOM
systems in enabling materials circularity practices.
This methodology combines theoretical development
with empirical exploration to identify key research
gaps. First, we explored theoretical background based
on selective literature review on the topics
‘manufacturing information systems’, ‘materials
circularity’, ‘circular supply chains’ and
‘manufacturing operations management’. We
prioritized recent studies from 2018-2025. The
review results highlighted a gap in literature on
integration of MOM systems with Industry 4.0
technologies to enhance traceability for materials
circularity. These literature findings were supported
by the subsequent empirical analysis.
To explore this gap, we used an in-depth
exploratory case study approach, drawing on
established methodologies by Voss (2002) (Voss,
2010) and (Yin, 2014). The study focused on two
large global manufacturing companies, anonymized
as Company A and Company B for confidentiality.
Both companies operate at scale with complex
material requirements in their production processes.
The case study methodology followed the six-stage
framework proposed by (Flynn et al., 1990). Data
collection included field visits involving direct
observations and discussions with manufacturing
personnel, supply chain managers, and sustainability
officers. We also reviewed publicly available
company reports, operational manuals, and
regulatory compliance records. To contextualize and
Industry 4.0 Information Systems for Materials Circularity in Supply Chains: Industry Issues and Research Directions
927

triangulate the data, we integrated insights from the
existing literature.
To ensure validity, we applied a theoretical
replication strategy, using Company B to confirm
findings and explore variations in materials
circularity needs under different operational contexts.
Reliability was maintained through data
triangulation, combining insights from literature,
field visits, and archival documentation. Preliminary
case study analysis contributed to the development of
hypotheses regarding the role of MOM systems in
achieving materials circularity goals.
This approach helped us in presenting the case
study findings in Section 4.
4 ANALYSIS AND FINDINGS
In this section, we present two exemplar case study
narratives of companies on their material circularity
journeys. These international companies have a large
manufacturing footprint and are industry leaders in
their respective sectors, known for their iconic
products.
Case 1 – High end Plastic Consumer Goods: A
global leader in plastic consumer goods
manufacturing, this company boasts an iconic product
and nearly a century of industry presence. With a
widespread manufacturing network spanning five
sites across three continents, it has adaptable supply
chains and an e-commerce platform to cater to the
global demand for personalized products. Company
A offers over 3,000 distinct shapes of components in
a wide range of configurations which are durable for
a long functional lifetime and often passed on into
secondary market. In terms of reuse/recycling this
also means that many generations of material
composition can be found in active market use, some
of which also contain substances that do not live up
to current requirements. This introduces a challenge
in terms of sorting products when they return through
take back programs so that unwanted substances are
not mixed into the material pool.
Company A has for 20 years been actively
engaged in changing the composition of its materials
platform and is under increasing internal and external
pressure to adopt sustainable practices, particularly in
terms of reducing plastic waste and improving
product recyclability.
In the 2010s, Company A integrated a new
comprehensive PLM system into its enterprise
platform to accelerate product launches and enhance
master data management across its supply chain. This
integration has driven increased automation in
product launches, production planning, control, and
lifecycle management, resulting in a notable
improvement in product output through more
granular control over materials, production processes,
and product flows. The upgraded data systems have
provided deeper insights into costs and
manufacturing structures, facilitating more informed
decision-making. A recent initiative focuses on
experimenting with dynamic recipes to align material
composition, process parameters, and advanced
customer requirements. Initially aimed at boosting
process control and optimization, this program, as it
evolves, will enable more significant changes in
material composition, processes, and customization.
These changes, whether process optimizations or new
elements, often require extensive testing and
validation, but digital twins within the industrial
metaverse now serve as a central hub to integrate
operational systems into a unified virtual
environment. For example, recipe adjustments can be
simulated and automatically updated across
connected systems.
Case 2 – Pharmaceuticals and Health Care
(Packaging): Company B is a global leader in the
pharmaceutical industry, known for its commitment
to fighting chronic diseases by advancing innovative
medicines and delivery systems. It is a well-
established company operating a vast network of
manufacturing sites across multiple continents.
Company B is also known for its pioneering work in
medicine delivery systems, where it has a self-
reported plastic footprint of 0.35kg per patient despite
concerted efforts to reduce consumption by, among
other things, introducing reusable delivery systems.
Company B faces the challenge of recycling its
plastic medical devices while adhering to stringent
health and safety regulations, which means that
closed loop solutions are not possible under current
circumstances, complicating efforts to improve
product recyclability and reduce plastic waste.
Compared to Company A, ensuring the right
functional properties of the material is not sufficient
for Company B as the documentation trail also need
to comply with regulatory regimes in the market.
Recycled materials often exhibit inconsistent
properties, such as variability in composition,
contamination risks, and reduced mechanical
integrity, which can compromise product safety and
sterility. Regulatory frameworks, including Food and
Drug Administration (FDA) and EU guidelines,
require traceability and compliance, complicating the
use of recycled plastics. Additionally, the
ICEIS 2025 - 27th International Conference on Enterprise Information Systems
928
compatibility of recycled plastics with sensitive
pharmaceutical products, such as drugs and medical
devices, remains uncertain. Ensuring uniformity,
maintaining compliance, and addressing consumer
safety concerns demand advanced sorting, cleaning,
and testing technologies, which increase costs and
complicate adoption. The company has implemented
a take-back program for packaging and dispensing
devices, connecting it to a recycling network for high-
value repurposing. While legislation and regulations
currently prevent the company from reusing materials
internally, it is progressing towards closed-loop
recycling, supported by material verification and
detailed process tracking to meet regulatory
requirements for materials not directly in contact with
active ingredients.
We analyze the empirical data from the two
companies and triangulate it with archival documents
and industry reports. This data is used to identify
specific requirements and challenges, which are
summarized in Table 1.
Table 1: Summary of collected qualitative data.
Source Primary data
from Company
A
Primary data
from Company B
(to ensure
reliability)
Size
(employees)
>10,000 >10,000
Industry High end plastic
consumer
g
oods
Pharmaceuticals
Field stud
y
Denmar
k
Denmar
k
Finding 1
Context
Dynamic recipes
(mixability,
additives,
compounding)
Complying with
industry
standards and
regulation (e.g.,
FDA)
Finding 2
Aim
Recycled content
in core product,
increased
customization,
and process
control for
optimization
Recycled content
in primary and
secondary
packaging
Finding 3
Problem
Meeting high
level functional
demands of
products
Documentation
trail to meeting
high standards
and demand from
re
g
ulator
y
bodies
Both companies face significant challenges in
adapting to changing regulatory requirements. This
highlights the need for advanced information systems
in supporting circularity via data visibility. We argue
that MES/MOM has the potential to address these
issues through dynamic recipes. Dynamic recipes
refer to production instructions or formulations that
adjust based on variables such as material quality,
equipment conditions, or batch requirements.
MES/MOM can handle this by dynamically updating
work instructions, equipment settings, and quality
checks during production.
MES/MOM Functionalities Supporting Circular
Economy:
• MES/MOM supports dynamic recipes
through ongoing alignment of material
characteristics, machine settings, and market
demands. MES/MOM manages and
executes production orders on the shop
floor, often interfacing with real-time data
from equipment and sensors.
• It supports the real-time alignment of actual
circumstances in production and the material
usage by recording the production data in
real-time.
• It enables material documentation and
maintains verified material histories by
documenting material properties and
minimizing the need for additional material
testing.
• It ensures traceability and compliance by
recording recipe changes and their impact on
the final product.
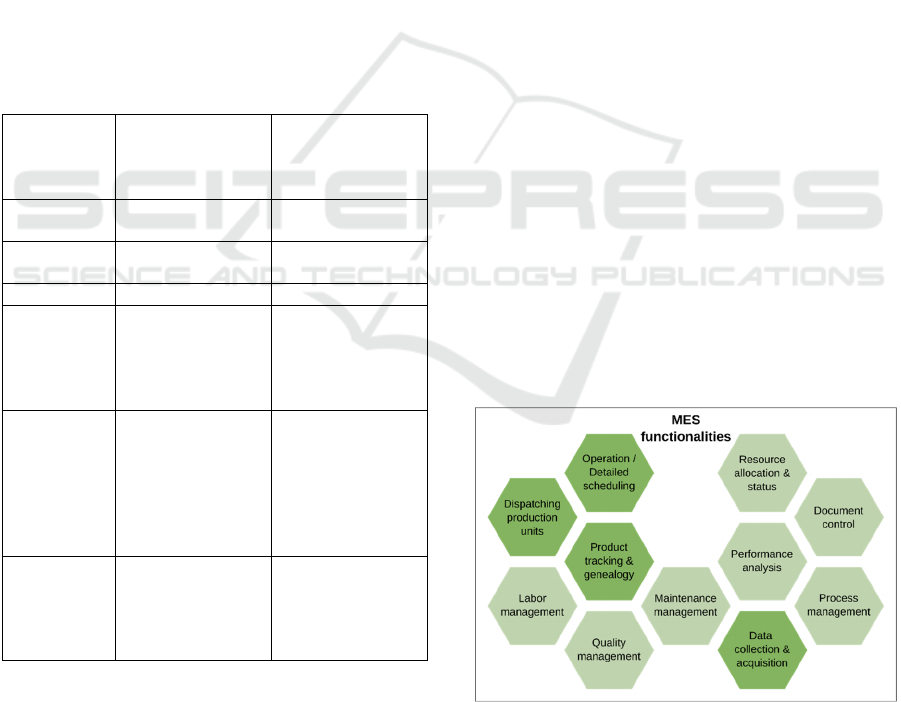
In Figure 2, we present the functionality diagram of
MES/MOM and highlight the functionalities that
could address the materials circularity issues in
manufacturing supply chains through its integration
with other enterprise systems and information
visibility.
Figure 2: 11 functionalities of MES/MOM according to
(MESA INTERNATIONAL – WHITE PAPER NUMBER
2, 1997)
Industry 4.0 Information Systems for Materials Circularity in Supply Chains: Industry Issues and Research Directions
929

5 CONCLUSIONS & FUTURE
RESEARCH DIRECTIONS
Our study explored how manufacturing operations
management (MOM) systems could enhance
traceability across the supply chain to support
materials circularity practices in manufacturing. By
analyzing the aims and processes of our case
companies, we identified the relevance of MOM
functionalities, such as dynamic recipes, which
allow production processes to adapt to varying inputs
or conditions. While traditionally utilized in the
process industry, dynamic recipes highlight the
potential of MOM systems to support materials
circularity across diverse manufacturing sectors.
Furthermore, the integration capabilities of MOM
systems with other enterprise systems, such as
enterprise resource planning (ERP) and product
lifecycle management (PLM), provide opportunities
to align production practices with material circularity
principles.
The key contributions of this paper include:
• We provided a perspective on conducting
high-impact research for complex
manufacturing supply chains to meet
evolving regulatory demands. MOM
systems were shown to effectively track
material flows, enabling manufacturing
personnel to monitor product lifecycle
details and improve recycling and reuse
rates. Recognizing the socio-technical and
organizational aspects of information
systems, we advocate for future research to
explore MES/MOM usability and their
benefits in supporting circular economy
principles.
• Potential in MES/MOM: We highlight the
need for Industrial IoT-based traceability.
Future research on this topic should focus on
the integration of IIoT-enabled traceability
within MOM systems to better align with
regulatory requirements and sustainability
targets, particularly in international
manufacturing networks.
• The study highlights the limited attention in
existing literature regarding MES/MOM
benefit realization for scalable solutions to
achieve material circularity goals. This
paper contributes by emphasizing the need
for further exploration in this area.
• Establishing a research agenda: A concept
was developed to guide future research,
suggesting that the integration of
information systems, especially Industry 4.0
compliant MES/MOM in manufacturing
operations can significantly improve supply
chain visibility and facilitate compliance
with circular economy principles.
As far as we know, this is the first study to
combine the topic of MES/MOM with material
circularity solutions in manufacturing. Most studies
on Industry 4.0 for circular supply chains focus on
IoT and AI but overlook enterprise information
systems like MES/MOM. Future research should
explore how these systems can support business
models such as Product-as-a-Service and take-back
schemes alongside Industry 4.0 adoption. This study
contributes to directives like the EU Green Deal and
the UK’s Net Zero goals.
Our position within the context of Industry 4.0
principles highlights the key capabilities of MOM
systems, such as real-time adjustments, traceability,
and interconnection, as enablers of circular economy
practices. As extended producer responsibility
policies tighten, manufacturers need real-time
material traceability. While MES/MOM can facilitate
this, challenges remain, including integration with
legacy systems, data standardization, cybersecurity
risks, and high AI implementation costs, highlighting
the need for further research on enabling
technologies.
Future work will include in-depth case studies to
explore how leveraging MOM systems for materials
circularity can be most effective when supported by
robust supply chain partnerships and real-time data
sharing. This ongoing research aims to deepen
understanding and provide actionable insights for
designing sustainable manufacturing operations
aligned with Industry 4.0 and circular economy goals.
REFERENCES
Awan, U., Sroufe, R., & Shahbaz, M. (2021). Industry 4.0
and the circular economy: A literature review and
recommendations for future research. Business Strategy
and the Environment, 30(4), 2038–2060.
Chhimwal, M., Agrawal, S., & Kumar, G. (2022).
Challenges in the implementation of circular economy
in manufacturing industry. Journal of Modelling in
Management, 17(4), 1049–1077.
Dumée, L. F. (2022). Circular materials and circular
design—review on challenges towards sustainable
manufacturing and recycling. Circular Economy and
Sustainability, 2(1), 9–23.
European Union. (2015). An action plan for the Circular
Economy. Communication from the Commission to the
ICEIS 2025 - 27th International Conference on Enterprise Information Systems
930

European Parliament, the Council, the European
Economic and Social Committee and the Committee of
the Regions Closing the Loop. Brussels, 2.
Flynn, B. B., Sakakibara, S., Schroeder, R. G., Bates, K. A.,
& Flynn, E. J. (1990). Empirical Research Methods in
Operations Management. Journal of Operations
Management, 9(2), 250–284.
https://doi.org/10.1016/0272-6963(90)90098-X
Gebhardt, M., Kopyto, M., Birkel, H., & Hartmann, E.
(2022). Industry 4.0 technologies as enablers of
collaboration in circular supply chains: A systematic
literature review. International Journal of Production
Research, 60(23), 6967–6995.
MESA INTERNATIONAL – WHITE PAPER NUMBER
2. (1997). In MESA International (Issue 2).
Omair, M., Stingl, V., & Wæhrens, B. V. (2024). Circular
Economy of Plastic: Revisiting Material Requirements
Planning Practices for Managing Uncertain Supply.
Sustainability, 17(1), 112.
Scholten, B. (2007). The Road to Integration: A Guide to
Applying the ISA-95 Standard in Manufacturing. ISA.
Taddei, E., Sassanelli, C., Rosa, P., & Terzi, S. (2022).
Circular supply chains in the era of Industry 4.0: A
systematic literature review. Computers & Industrial
Engineering, 170, 108268.
Uhrenholt, J. N., Kristensen, J. H., Adamsen, S., Jensen, S.
F., Colli, M., & Wæhrens, B. V. (2023). Twin
Transformation: Synergies between Circular Economy
and Internet of Things: A study of Danish
Manufacturers. Circular Economy, 1(1).
Voss, C. (2010). Case research in operations management.
In Researching operations management (pp. 176–209).
Routledge.
Yin, R. K. (2014). Case Study Research: Design and
Methods (5th ed.). SAGE Publications Inc.
Zeiss, R., Ixmeier, A., Recker, J., & Kranz, J. (2021).
Mobilising information systems scholarship for a
circular economy: Review, synthesis, and directions for
future research. Information Systems Journal, 31(1),
148–183.
Industry 4.0 Information Systems for Materials Circularity in Supply Chains: Industry Issues and Research Directions
931
