
Towards a Digital Twin of the Cardiovascular System
Ciro Nespolino
a
, Roberta De Fazio
b
, Laura Verde
c
and Stefano Marrone
d
Dipartimento di Matematica e Fisica, Universit
`
a degli Studi della Campania “Luigi Vanvitelli”,
viale Lincoln 5, Caserta, Italy
{ciro.nespolino, roberta.defazio, laura.verde, stefano.marrone}@unicampania.it
Keywords:
Human Digital Twins, Heartbeat Modelling, Ordinary/Partial Differential Equations, Cardiovascular System,
Recurrent Neural Network.
Abstract:
As medicine aims to become smarter, more pervasive, and more personalised, the concept of the Digital Twin
has become a cornerstone of the entire base and applied research. The advantages of having Digital Twins
to understand, predict and communicate complex mechanisms and functionalities have become of paramount
importance in modern and future medicine. This paper presents an approach for the construction of a Digital
Twin for the cardiovascular system. The approach, with the objective of being as lightweight and explainable
as possible, is based on the integration of partial differential equation models and of realistic data. This
integration can overcome both the rigidity of traditional model-based methods and the computational demands
of modern deep learning approaches. A technical integration of a smart backend with a frontend based on
virtual reality visor is presented in the paper.
1 INTRODUCTION
In a society where the use of high-tech devices in
daily life is accessible to everyone, various research
fields contribute to the ambitious goal of improving
life. The impact achieved by the Internet of Things
(IoT) massive employment in Cyber Physical Sys-
tems (CPSs) has been concretised into the availabil-
ity of large amounts of data, enabling the applica-
tion of sophisticated analysis techniques (Ramasamy
et al., 2022). In this framework, medical applica-
tions are acquiring a role of significant importance.
The availability of huge amounts of data from dif-
ferent sources contributes to having a holistic and
complete view of medical problems, supporting the
definition of more realistic predictive models. This
ambitious purpose, integrated into the real clinical
practice, is translated into the definition of innova-
tive Decision Support Systems (DSSs) that contribute
to achieve personalised medicine objectives (Marques
et al., 2024).
In the direction of personalised approaches to dis-
ease diagnosis and therapy, efforts are devoted to Ar-
tificial Intelligence (AI)-based techniques that use In-
a
https://orcid.org/0009-0003-8687-8614
b
https://orcid.org/0000-0002-0271-132X
c
https://orcid.org/0000-0003-2422-1732
d
https://orcid.org/0000-0003-1927-6173
ternet of Medical Things (IoMT) and wearable de-
vices to adapt models, training them on patient data
(Alshamrani, 2022). One of the principal strengths of
Data-Driven (DD) techniques is flexibility, intended
as the ability to tailor the model to the specific sce-
nario, extracting the knowledge directly from the data
and making the prediction more adherent to the real
practice (Yu et al., 2021).
In this context, Digital Twins (DTs) play a crucial
role. A DT is a virtual replica that monitors and inter-
acts with a twinned physical system to predict and re-
act to meaningful events, also aiming at an optimisa-
tion of the system itself (Campanile et al., 2023). One
of the characteristics, that contributes to the great suc-
cess achieved by DTs in the last decade, is its capabil-
ity to enable holistic views of a problem (Hemamalini
et al., 2024). This property allows the integration of
data and information from different sources: from the
vital parameters recorded by IoMT sensors and med-
ical diagnostic data — such as Magnetic Resonance
Imaging (MRI), Computed Tomography (CT) — to-
wards the long-term information regarding follow-ups
and therapies. Human Digital Twins (HDTs) are de-
signed to replicate the patient’s Health State (HS),
considering his/her interaction with the physical envi-
ronment and his/her responses to the treatments, en-
hancing and supporting personalised medicine.
The integrated view is not only limited to the in-
Nespolino, C., De Fazio, R., Verde, L. and Marrone, S.
Towards a Digital Twin of the Cardiovascular System.
DOI: 10.5220/0013520700003944
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 10th International Conference on Internet of Things, Big Data and Security (IoTBDS 2025), pages 481-490
ISBN: 978-989-758-750-4; ISSN: 2184-4976
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
481

formational sources, but also involves the analytics
approaches. The concept of DT is based on the ne-
cessity to establish a connection between the digi-
tal and physical world. In the literature, this task is
assessed by introducing a layered architecture, tradi-
tionally based on three interconnected layers (Hassani
et al., 2022; Okegbile et al., 2023; Wang et al., 2022).
In a previous work, we introduced a four-layers archi-
tecture for HDT to address the integration between
Model Based (MB) and DD approaches (De Fazio
et al., 2025). DD techniques are the key for exploit-
ing the value of data obtained by IoT network. De-
spite being flexible and accurate in the predictions,
the generated model is not fully explainable. In criti-
cal contexts, especially medical ones where some de-
cisions could impact patients’ HS, a clear view of
the mechanisms that guided the decisions is strictly
required. In other words, models’ explainability is
highly demanded in healthcare applications, reinforc-
ing the medical staff reliance in AI application to sup-
port the decision-making process (Antoniadi et al.,
2021). The integration of MB approaches, based on
a domain-aware formal definition of the system, ad-
dresses this point, providing a transparent view of the
model and including experts’ knowledge. This hy-
brid approach, known as Scientific Machine Learning
(SML), is raising interest in the scientific community
and, particularly, in clinical practice.
This work introduces the Differential Equation
baSed dIGital twiN (DESIGN) reference architecture
for the definition of Digital Twin of the Cardio Vascu-
lar System (CVS-DT), framed in SML paradigm. In
detail, the architecture is based on an Ordinary/Partial
Differential Equation (O/PDE) model and Machine
Learning (ML) approaches for adapting the model to
real-world data. The complexity of the O/PDE system
varies based on several factors. The Cardio-Vascular
System (CVS) is represented using a simplified theo-
retical model for clarity and transparency.
The proposed architecture can be used with dif-
ferent modelling and DD techniques. In this paper,
a more specific application for CVS-DT is presented,
exploiting a set of differential equations, introduced
by Zenker (Zenker et al., 2007), and the platform
based on the study of Linial et al. (Linial et al., 2021).
While this paper generalises the results of a previ-
ous work designed for heartbeat prediction (Marrone,
2024), it presents novel original contributions, here
summarised:
• presenting the DESIGN reference architecture for
the CVS, extending the previous paper, oriented
to the heartbeat prediction;
• using a more complex characterisation of the pa-
tient is possible, considering “systemic” parame-
ters such as pressure, Heart Failure (HF), etc.;
• capability of modelling medical actions as thera-
pies or drug administration, and the possibility to
view the effect of patient’s variables evolution.
Regarding these points, the paper is structured as
follows: Section 2 presents a brief review of the works
that encompass architectures and mathematical mod-
els underlying the definition of CVS-DTs; Section 3
reports the DESIGN reference architecture and detail
the role of architecture elements; Section 4 defines
the perspectives on how to conjugate the reference
architecture and the Generative ODE modeling with
Known Unknowns (GOKU) tool; Section 5 illustrates
client-server architecture based on the microservice
architectural pattern; Section 6 draws some conclu-
sion and future perspectives.
2 RELATED WORK
This section provides a brief review of the literature,
investigating two primary aspects adopted in the pre-
sented work: the development of a CVS-DT and the
mathematical modelling of the CVS in general.
2.1 Digital Twins of CVS
The adoption of DT for the cardiovascular system
will provide useful computational tools for both re-
search and clinical practice. However, this requires
reliable, well-defined models and methods for the dif-
ferent stages of the process (Coorey et al., 2022).
A Vascular Coordinate System (VCS) is presented
in (Romero et al., 2025). It provides a clear and pre-
cise method for defining positions in a vascular sec-
tion. The VCS model has been tested in several appli-
cations, including the development of a robust, low-
dimensional, patient-specific vascular model used to
study the phenotypic variability of the thoracic aorta
in a cohort of patients. Point correspondences were
used to construct a hemodynamic atlas of the aorta
based on fluid simulations using the Navier-Stokes
equations with the finite volume method.
While a Longitudinal Haemodynamic Mapping
Framework (LHMF) is proposed in (Tanade et al.,
2024), designed to capture personalised 3D blood
flow dynamics over a timescale of months. This re-
alised 3D coronary DT is continuously updated with
data from wearable devices. In addition, hemodynam-
ically similar heartbeats are grouped to minimise re-
dundant simulations and enable accurate reconstruc-
tion of Longitudinal Hemodynamic Maps (LHM).
The study described in (Hermida et al., 2024) uses
AI4EIoT 2025 - Special Session on Artificial Intelligence for Emerging IoT Systems: Open Challenges and Novel Perspectives
482

DTs to improve the understanding and prenatal diag-
nosis of Coarctation of the Aorta (CoA). A statisti-
cal model of the shape of the fetal aortic arch is con-
structed from cardiac MRI data of 188 fetuses. A DT
approach is proposed, which is capable of performing
Computational Fluid Dynamics (CFD) simulations
of the three-dimensional hemodynamics of the aor-
tic arch to predict specific biomarkers and outcomes.
In detail, analyses show that changes in arch shape
and left-right ventricular output balance resulted in
qualitatively similar haemodynamic changes. This
approach highlights the importance of a combined
anatomical and functional diagnosis in CoA.
A dynamic DT for healthcare, designed to op-
timise individual care pathways, particularly for
women at risk of cardiovascular complications, is pre-
sented in (Mulder et al., 2022). This DT evolves over
time, adapting to life conditions and patient needs,
such as fertility prevention or acute disease manage-
ment. Its dynamism makes it possible to update goals
and forecasts based on up-to-date data specific to each
stage of life. This capability to stay connected to
the real system is possible due to wearable devices
for continuous monitoring, enabling early interven-
tion and improving the relevance of predictions com-
pared to standard intermittent measurements.
The study (Chakshu et al., 2021) proposes, in-
stead, a DT-based methodology for inverse analysis
of the cardiovascular system using Recurrent Neural
Networks (RNN), using a virtual database of patients.
Blood pressure waveforms in different vessels are in-
versely reconstructed using Long Short-Term Mem-
ory (LSTM) models from non-invasive measurements
on the carotid, femoral and brachial arteries. The sys-
tem is used to detect and assess the severity of Ab-
dominal Aortic Aneurysms (AAA). Data from acces-
sible sites are used to predict pressure in other areas,
and a Neural Network (NN) model analyses these pre-
dictions to identify and characterise aneurysms.
2.2 CVS Mathematical Models
CVS mathematical and numerical modelling has at-
tracted considerable interest from the research com-
munity over the last 25 years. In this context, several
studies exist in the literature. In (Quarteroni et al.,
2017) an in-depth review of the main mathematical
modelling of the CVS is presented. In detail, several
models, describing arterial circulation and heart func-
tion with its electrical and mechanical activities, are
presented.
An adaptive step method is proposed in (Garc
´
ıa-
Moll
´
a et al., 2014) for large Ordinary Differential
Equation (ODE) systems on Graphics Processing
Units (GPUs) for simulating electrical cardiac activ-
ity. The study compares the performance of the pro-
posed adaptive methods with fixed-step methods and
finds that while fixed-step methods can achieve higher
speed, adaptive-step methods demonstrate superior
accuracy and robustness.
A multiscale approach is presented in (Lagana
et al., 2005), which is, instead, designed to prescribe
appropriate and realistic boundary conditions for the
3D model of the circulation following the Norwood
procedure. This method enhances a more accurate
reproduction of realistic conditions compared to the
classical approach, allowing the monitoring of both
local and global haemodynamics.
A scientific machine learning approach to con-
structing a comprehensive surrogate model that in-
tegrates cardiac and cardiovascular functions is pre-
sented in (Salvador et al., 2024). This method
involves training a system of Latent Neural Ordi-
nary Differential Equationss (LNODEs) to learn the
pressure-volume transients of a HF patient while
varying 43 model parameters. These parameters cap-
ture cardiac electrophysiology, active and passive me-
chanics, and cardiovascular fluid dynamics. The
training uses 400 3D-0D closed-loop electromechan-
ical simulations. The LNODEs framework enables
global sensitivity analysis and parameter estimation
with uncertainty quantification, completing the pro-
cess within 3 hours of computation on a single pro-
cessor.
3 THE DESIGN APPROACH
This section outlines the proposed approach, starting
from a reference scenario. In this scenario, a doctor
and a patient are interacting: the patient is monitored,
to retrieve his/her vital parameters, and the doctor de-
cides which is the best action to perform for the pa-
tient’s health, according to his/her HS. The main aim
of the proposed architecture is to provide a system
that could be:
• R1: fed by the patient’s current data;
• R2: queried by the doctor to understand the pos-
sible evolutions of the patient’s HS;
• R3: used as a what-if tool by the doctor to “test
in-silico” the effect of possible actions (e.g., inter-
ventions, drug administration).
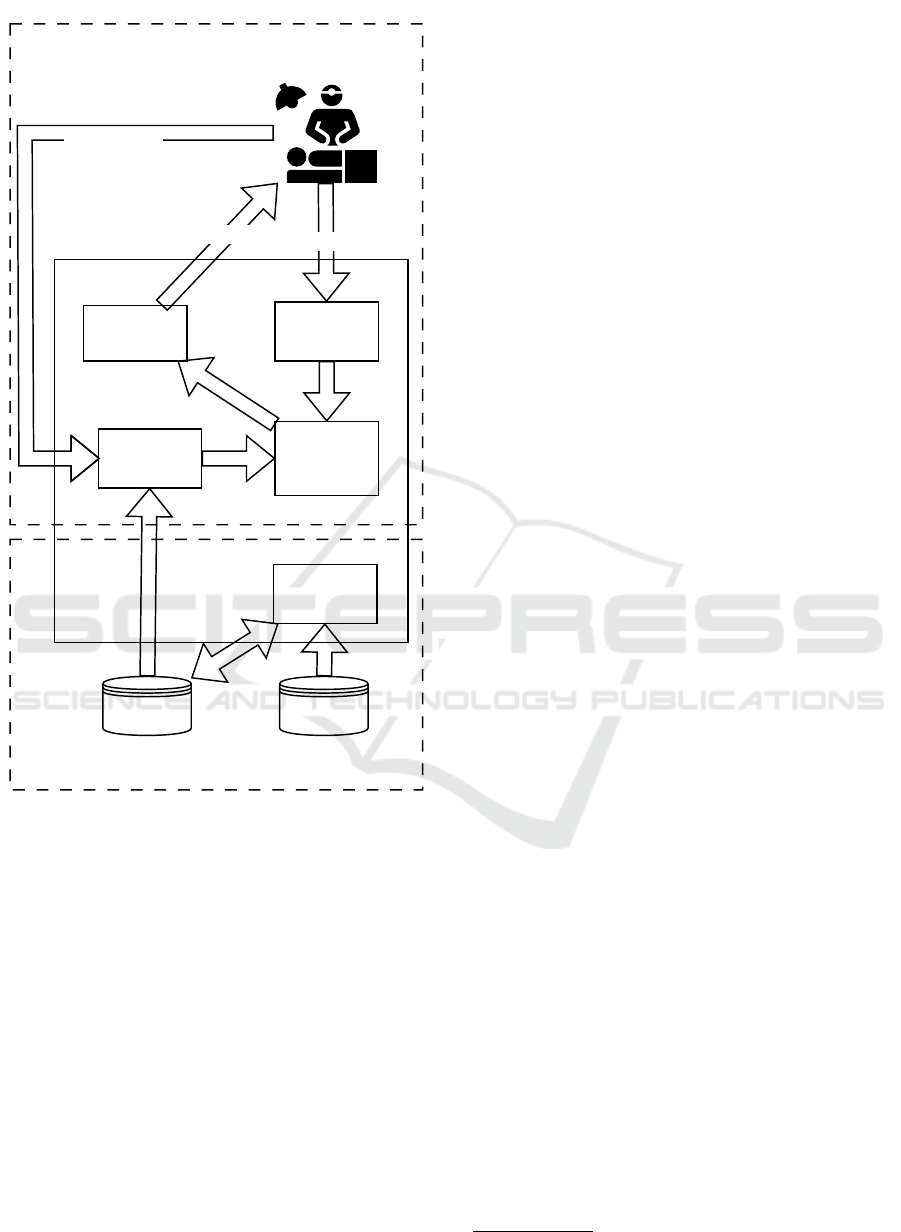
Figure 1 presents a reference architecture of the
DESIGN approach.
At the centre of the approach, there is the defini-
tion of a set of O/PDE models, which are stored in the
Towards a Digital Twin of the Cardiovascular System
483
on-line plane
off-line plane
DESIGN
Patient &
Doctor
Model
Repository
Patient
Data
O/PDE
Evaluator
Model
Learning
Model
Selection
Parameters
and Variables
Variables
Model
Perturbation
Action
Figure 1: The overall approach.
Model Repository. These models describe the evo-
lution in time of a set of variables, X , subject to the
value of some parameters, P . It is worth underlining
that the nature of these models is not strictly bounded
to the O/PDE, since other possible forms fit in the DE-
SIGN approach (e.g., ML or Petri Net (PN) models,
or hybrid approaches).
The approach is then structured of two different
planes, named off-line plane and on-line plane. The
off-line plane is used to tune the models to obtain
a usable Model Repository, while the on-line plane
uses the trained models, choosing the proper values of
the parameters in P , and interacting with the doctor-
patient scenario.
The Off-Line Plane. The off-line plane set of ele-
ments contains a tool, the Model Learning, and two
repositories, Patient Data and Model Repository. By
collecting and pre-processing patients’ historical data,
the Model Learning tool is responsible for analysing
data and generating — also starting from pre-existing
partial models stored in the Model Repository — one
or more models describing the phenomenon under
study. Once a model is generated by fitting the data,
it is stored in the Model Repository.
The On-Line Plane. The on-line plane is responsi-
ble for setting up, running and evaluating the output
of the DT. Once the patient’s monitored data is gen-
erated, both parameters and variables are used to un-
derstand which model, in the Model Repository, ade-
quately fits the data. This task is responsible for the
Model Selection block, which produces the O/PDE
model. Once the model is chosen and tuned with the
specific features of the patient, the Evaluator analyses
the model and provides the doctor the possible future
evolution of the patient’s HS, by computing model
variables. As a last step, the doctor could define a
possible treatment plan to improve the patient’s HS by
supposing one or more actions to perform; the Model
Perturbation block affects the parameters and/or the
variables of the model and allows the doctor to under-
stand the effect of his/her hypotheses.
The O/PDE Model Structure To provide a de-
tailed technical description of the approach, this sec-
tion outlines the following model structure.
Let x(t) =< x
1
(t), x
2
(t),... ,x
n
(t) > denote the
variables of the O/PDE system, referred to as the
Variable Vector. Its temporal evolution explicitly
depends on a set of parameters characteristic of the
phenomenon under investigation, collected in the
vector p(t) =< p
1
(t), p
2
(t),... , p
m
(t) >, referred to
as the Parameter Vector. This dependence can
be expressed as a vector of functional relationships
f
P
(t; x(t)), referred to as the Dynamics Function.
When the vector p(t) is set, it defines a specific con-
figuration of the phenomenon under study (e.g., in
case of the CVS, this could represent a particular
heart condition or a heart undergoing a specific med-
ical treatment). Once a configuration is defined, the
goal is to estimate the temporal evolution of the phe-
nomenon through the integration of the O/PDE sys-
tem.
This function is explicated in a system of k dif-
ferential equations regarding the time variable t, as
shown in Eq. 1
1
:
1
This system is based on the hypothesis that k ≤ n. The
AI4EIoT 2025 - Special Session on Artificial Intelligence for Emerging IoT Systems: Open Challenges and Novel Perspectives
484
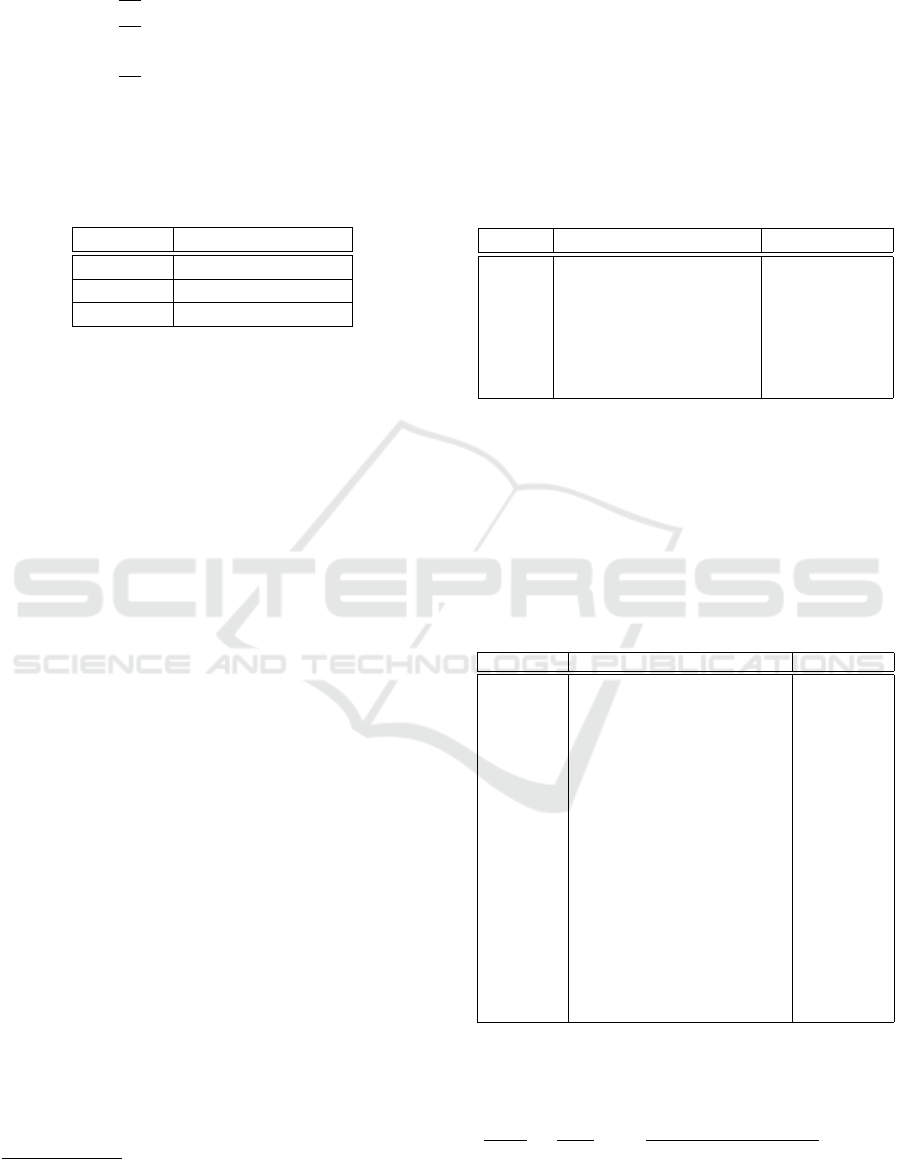
dx
1
dt
= f
1,P
(t; x
1
,x
2
,... ,x
n
)
dx
2
dt
= f
2,P
(t; x
1
,x
2
,. .. ,x
n
)
.. .
dx
k
dt
= f
k,P
(t; x
1
,x
2
,. .. ,x
n
)
(1)
The three presented blocks map the three func-
tionalities described at the beginning of this section
(see Table 1).
Table 1: Mapping between functionalities and blocks.
Use Case Block
R1 Model Selection
R2 Evaluator
R3 Model Perturbation
In Section 5, a concrete architecture is reported,
and its realisation is described. While the reported
example and the entire paper are devoted to the CVS,
it is clear that the overall approach described in this
section can be extended also to other systems (e.g.,
renal system, hepatic system).
4 BUILDING A
CARDIOVASCULAR SYSTEM
DIGITAL TWIN
This section shows how the general approach pre-
sented in Section 3 can be customised into Differen-
tial Equation baSed dIGital twiN for Cardio-Vascular
System (DESIGN
CVS
) for the realisation of a CVS-
DT, possibly exploiting existing platforms. In partic-
ular, the GOKU approach presented in (Linial et al.,
2021) has been chosen as the best candidate for real-
ising DESIGN
CVS
.
Hence, this section is structured as follows:
• the O/PDE model underlying this example, which
is the one presented by Zenker (Zenker et al.,
2007), is presented in Subsection 4.1;
• the GOKU approach and workflow and the adap-
tation of GOKU to the DESIGN
CVS
are presented
in Subsection 4.2;
• the execution of the experiments and the discus-
sion of the results in the proposed demonstration
are presented in Subsection 4.3.
system can be completed by other n − k equations, needed
to uniquely determine the solution, and that can be of a dif-
ferent nature (e.g., non-differential).
4.1 The Zenker’s -ODE Model
As outlined in Section 2, significant research has been
conducted on the development of a O/PDE system to
represent the functioning of the CVS. This approach
is built upon the proposal by Zenker et al. (Zenker
et al., 2007). It introduces a simplified representation
of the CVS focused on monitoring key variables, re-
ported in Table 2, according to patient parameters.
Table 2: Variables used in the CVS O/PDE.
Name Description Unit
P
a
Artherial Pressure mmHg
P
v
Venous Pressure mmHg
S Baroreflex’s response —
S
V
Stroke volume ml
R
T PR
TPR value mmHg · s/ml
f
HR
Heart Rate (HR) value Hz
Consequently, x(t) = x
O
(t)?x
H
(t) means that the
Variable Vector is constituted by two sub-vectors con-
catenated by the ? operator. More in detail, x
O
(t) =<
P
a
(t), P
v
(t), f
HR
(t) > refers to the variables in X
O
while x
H
(t) =< R
T PR
(t), S(t), S
V
(t) > refers to the
variables in X
H
.
Table 3, instead, describes the Parameter Vector
and the meaning of each parameter.
Table 3: System Parameter Vector used in the CVS O/PDE.
Parameter Description Unit
K
width
Baroreflex curve’s slope -
τ
baro
Baroreflex’s response time s
T
sys
Time of systolic phase s
f
HR
min
HR min value Hz
f
HR
max
HR max value Hz
I
external
External blood flow ml/s
V
ed,0
Initial telediastolic volume ml
S
V
Mod
Possible modification in S
V
ml
K
elv
Ventricular compliance constant 1/ml
P
a,set
Baroreflex target pressure mmHg
cprsw
min
PRSW min slope mmHg
cprsw
max
PRSW max slope mmHg
P
v,0
Initial venous pressure mmHg
C
a
Arterial compliance ml/mmHg
C
v
Venous compliance ml/mmHg
R
valve
Atrial valve resistance mmHg · s/ml
R
T PR
min
TPR min value mmHg · s/ml
R
T PR
max
TPR max value mmHg · s/ml
R
T PR
Mod
Possible modification in R
T PR
mmHg · s/ml
The specific model is reported in three equations,
Eq. 2, Eq. 3, and Eq. 4.
dS(t)
dt
=
1
τ
baro
1 −
1
1 + e
−k
width
(P
a
(t)−P
a,set
)
− S(t)
(2)
Towards a Digital Twin of the Cardiovascular System
485
R
T PR
(t) = S(t)(R
T PR
max
− R
T PR
min
) + R
T PR
min
+ R
T PR
Mod
f
HR
(t) = S(t)( f
HR
max
− f
HR
min
) + f
HR
min
(3)
dS
v
(t)
dt
= I
external
dP
a
(t)
dt
=
1
C
a
P
a
(t)−P
v
(t)
R
T PR
(t)
− S
v
(t) · f
HR
(t)
dP
v
(t)
dt
=
1
C
v
−C
a
dP
a
(t)
dt
+ I
external
(4)
This specific O/PDE model has an appropriate in-
tegration process, which is expressed in the following
steps:
1. a solution of S(t) is found (Eq. 2);
2. R
T PR
and f
HR
(t) are easily computed (Eq. 3);
3. the rest of the equations are solved (Eq. 4).
4.2 Implementing the DESIGN
CV S
Approach
The GOKU approach aims to integrate the defined
O/PDE system into a hybrid DD architecture to fore-
cast the CVS’s HR value (Linial et al., 2021). This
mixing of methods is conformant with the main ob-
jective of that work, which is to cope with uncertainty
in the estimation of the whole parameter vector as
well as the sub-vector of the hidden variables.
More in details, the GOKU architecture employs
a Virtual Auto Encoder (VAE) model, with the aim to
infer part of the model from observable variables:
• learning the patient’s HS in terms of initial con-
ditions x
O
(t
0
) of the O/PDE system, its specific
parametrisation p(t
0
), and the relationship be-
tween the system’s solution x
O
(t
1
) with the final
output x
H
(t
1
);
• the input of the VAE stage consists of a triplet of
values x
O
(t
0
), which is used to compute the initial
state of the O/PDE system, including the System
Parameter Vector P ;
• subsequently, the O/PDE system is solved, and the
solution is used to infer the triplet x
O
(t
∗
).
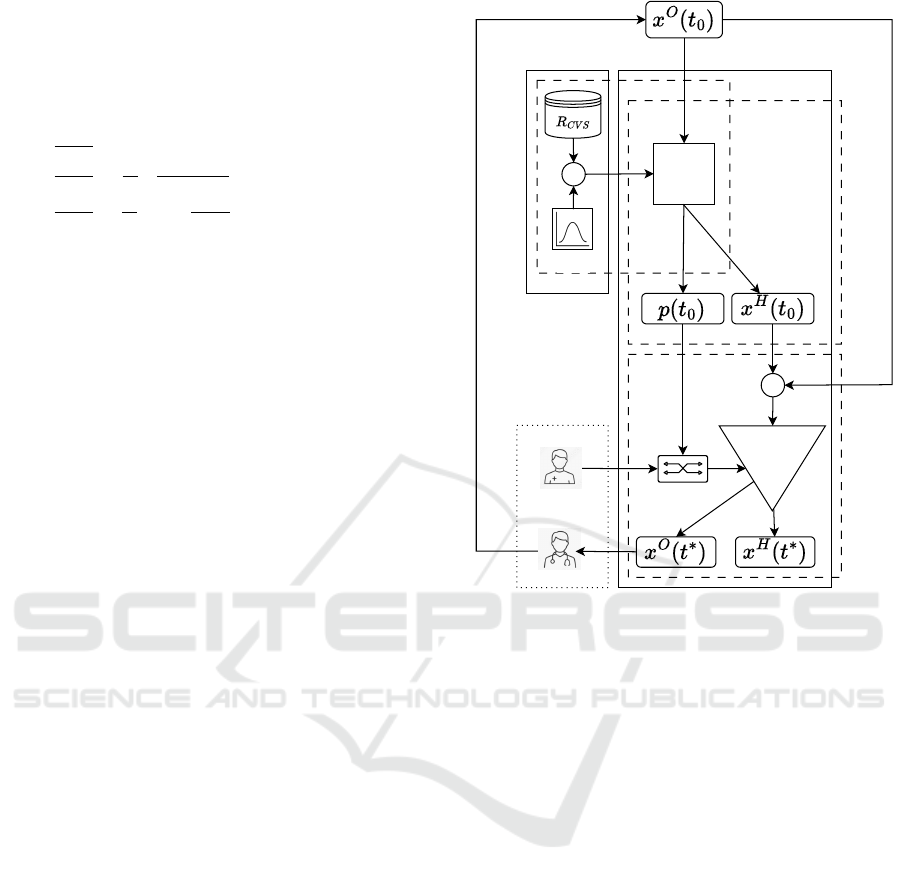
Starting from this background knowledge, the
DESIGN architecture “instantiation” on CVS here
proposed is shown in the Figure 2, offering the ad-
vantage of predicting the future state by integrating
the previously defined O/PDE system and exploiting
some of the GOKU components.
With respect to the three phases of the GOKU
approach, DESIGN maps each phase into one of its
components:
Patient & Doctor
on-line planeoff-line plane
Model
Learning
Feature
Extractor
Block
O/PDE
Solver
Block
Noise
+
?
Model
Selector
Evaluator
Figure 2: DESIGN architecture representation.
• the Model Learning starts from the definition of a
relation between the sets P and X with the specific
objective of representing the dataset (see Eq.5).
R
CVS
⊆ X
O
× X
H
× P (5)
As this dataset can be synthetic, noise could be
added as well, to let the training of a Feature Ex-
tractor Block.
• the Model Selector is based on this Feature Ex-
tractor Block, being able to infer from observable
variables x
O
(t
0
) at a certain instant of time t
0
. This
block extracts the parameters p(t
0
) and the hidden
variables x
H
(t
0
) at t
0
.
• the Evaluator exploits the ODE Solver Block. This
block gets as input the given initial observed vari-
ables x
O
(t
0
) as well as the initial inferred hidden
variables x
H
(t
0
), under the configuration deter-
mined by p(t
0
). The ODE Solver Block, by im-
plementing the solution process reported in Sub-
section 4.1. Once the ODE model is solved, the
time series x
O
(t
∗
) and x
H
(t
∗
) with t
∗
> t
0
are gen-
erated. x
O
(t
∗
) is then reported to the DT user.
In addition, DESIGN
CVS
incorporates a dual adap-
tation mechanism to address the following needs:
• Patient Adaptation: over time, a patient’s HS may
change (e.g., due to the onset of haemorrhage).
AI4EIoT 2025 - Special Session on Artificial Intelligence for Emerging IoT Systems: Open Challenges and Novel Perspectives
486
This adaptation mechanism is implemented con-
sidering the feedback from the DT user to x
O
(t
0
).
• Medical Intervention: medical staff may intervene
after analysing the patient’s vital parameters (e.g.,
by administering a treatment). This mechanism is
embedded in the change of the parameters by the
DT user. Such changes are then mixed with the
original parameter to determine the configuration
of the ODE Solver Block.
4.3 Use Case and Discussion
This subsection presents a first attempt of integrating
between DESIGN
CVS
and GOKU. To accomplish this
task, three distinct CVS HSs have been detected. In
detail, the Healthy config, which represents the CVS
of a healthy patient, the Unhealthy config, which rep-
resents the CVS of a patient with mild HF, and the
Bleeding config, representing the CVS of a patient
during a haemorrhage, are considered. Table 4 reports
the configuration’s details, while the parameter values
for each configuration are shown in Table 5.
Table 4: Sample CVS configurations.
ID Description
C
HE
This is the configuration corresponding to
a healthy patient.
C
UH
This is the configuration corresponding to
an unhealthy patient, suffering from mild
HF.
C
BL
This is the configuration corresponding to
a patient, who is bleeding due to some
trauma or other causes.
A simulation process, inspired by the approach in
(Linial et al., 2021), was used to generate a dataset
consisting of 1000 samples, each representing a dif-
ferent patient. Each sample is composed of a time
series of length 400 seconds, capturing the temporal
evolution of key vital parameters, including P
a
, P
v
,
S
V
, and f
HR
(see Table 6).
Then, the GOKU prototype is used to simulate
these CVS configurations. The results obtained from
the simulations are summarized in Table 7.
As reported in Table 7, x
O
(t
∗
) has been accurately
defined, demonstrating an appropriate response to the
proposed configurations. Specifically:
• P
a
in C
HE
exhibits an average trend around 78.8
mmHg, corresponding to a healthy patient. It
slightly increases to approximately 97.6 mmHg
in C
HF
, which represents a patient with mild HF.
Moreover, it significantly drops to 70.7 mmHg in
C
BL
, simulating a severe haemorrhage;
Table 5: Parameter Vector for each configuration.
Parameter C
HE
C
UH
C
BL
Unit
C
a
4 - - ml/mmHg
C
v
111.11 - - ml/mmHg
R
valve
0.0025 - - mmHg · s/ml
τ
baro
20 - - s
P
a,set
70 - - mmHg
K
width
0.1838 - - —
cprsw
min
25.9 - - mmHg
cprsw
max
103.8 - - mmHg
f
HR
min
0.6 1.2 - Hz
f
HR
max
3.1 - - Hz
R
T PR
min
0.5 0.6 - mmHg · s/ml
R
T PR
max
2.1 - - mmHg · s/ml
P
v,0
2.03 - - mmHg
V
ed,0
7.14 - - ml
T
sys
0.267 - - s
K
elv
0.066 - - 1/ml
S
V
Mod
0 0.005 0.01 ml
R
T PR
Mod
0 -0.2 - mmHg · s/ml
I
external
0 - -0.2 ml/s
Table 6: Few rows of the dataset simulated inDESIGN
CVS
architecture.
Patient Id Time P
a
P
v
S
v
S f
HR
Conf Id
1 1 107 5.50 94.6 0.0196 71.6 C
UH
1 2 95.8 4.63 94.6 0.0188 73.7 C
UH
... ... ... ... ... ... ... ...
1000 399 82.7 4.98 94.9 0.165 58.2 C
HE
1000 400 72.1 6.38 94.9 0.165 63.1 C
HE
• P
v
does not show substantial variations across the
three configurations. However, in the cases of a
healthy patient and a patient with severe haem-
orrhage, similar trends to those observed in P
a
monitoring can be identified (1.30 mmHg, 1.37
mmHg). In contrast, for the patient with mild HF,
a value close to zero is observed (0.780 mmHg);
• f
HR
is particularly sensitive to the defined config-
uration. As expected, it remains within a normal
range of around 60.8 bpm for a healthy patient, in-
creases slightly for a patient with mild f
HR
(72.8
bpm), and rises exponentially for a patient suffer-
ing from severe haemorrhage (95.6 bpm).
These variations follow a trend consistent with
the physiologically realistic f
HR
models expected for
both healthy and non-healthy patients, as illustrated
in Figure 3a. Figure 3b and Figure 3c respectively
present the results for P
a
and P
v
.
Some limitations, arising from the use of the
GOKU framework, have clearly shown the path for
a complete integration between the two approaches.
As GOKU does not explicitly support a change in pa-
rameter’ evolution, a modification of the mechanism
for getting such parameters is due. Furthermore, the
main mechanism of GOKU must be integrated into a
Towards a Digital Twin of the Cardiovascular System
487
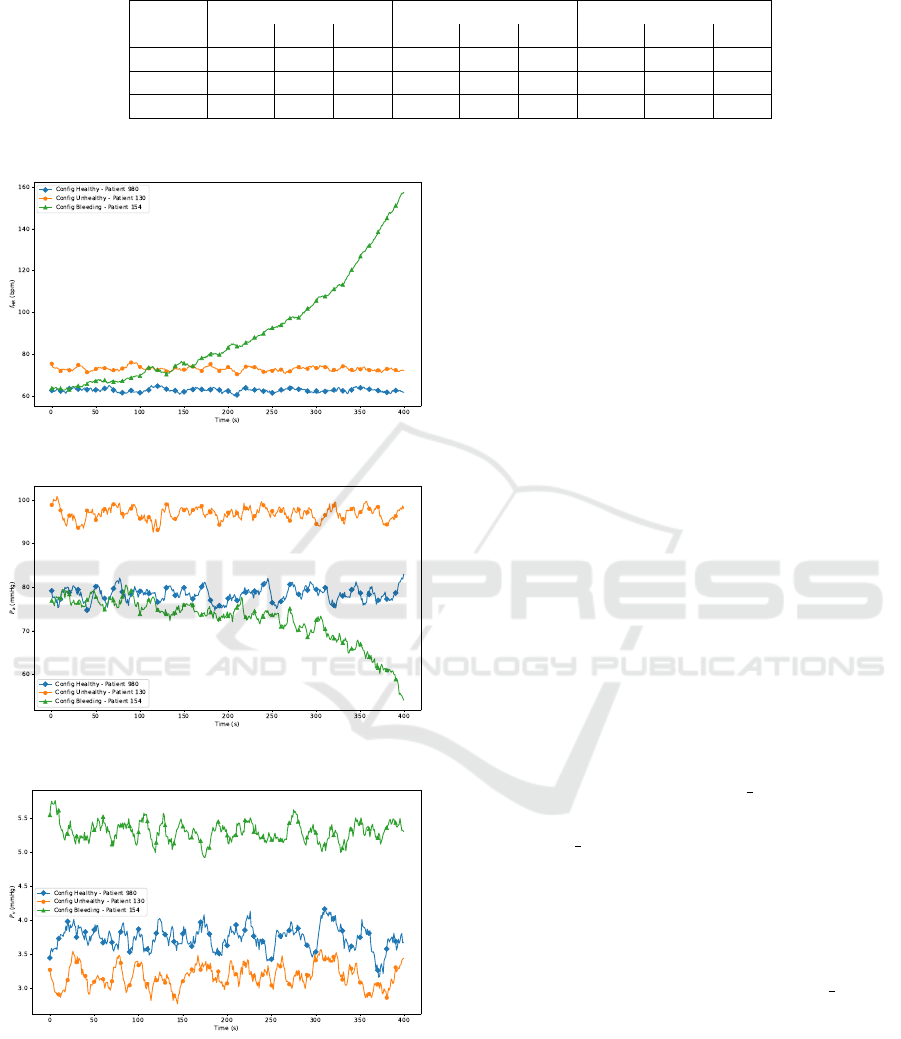
Table 7: Result Summary.
Con f
id
f
HR
(bpm) P
a
(mmHg) P
v
(mmHg)
mean min max mean min max mean min max
1 60.8 47.2 73.9 78.8 53.8 102 5.14 1.30 8.81
2 72.8 59.8 86.7 97.6 72.9 121 4.39 0.780 8.20
3 95.6 52.3 186 70.7 5.61 99.2 4.88 1.37 9.01
(a) Heart Rate Trend.
(b) Arterial Pressure Trend.
(c) Venous Pressure Trend.
Figure 3: Plots of heart rate, arterial pressure, and venous
pressure.
continuous mechanism for patient’s monitoring, clos-
ing the loop of interaction between the DESIGN
CVS
architecture and the medical staff.
5 TOWARDS A CLIENT-SERVER
ARCHITECTURE
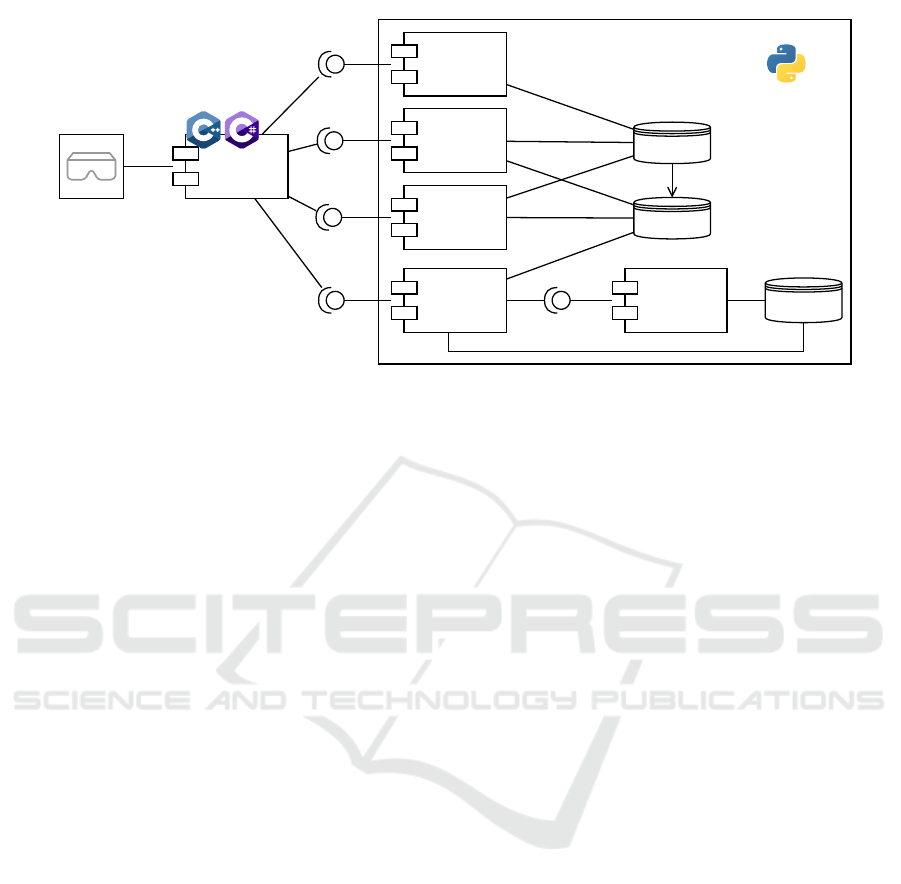
Figure 4 illustrates a client-server architecture based
on the microservice architectural pattern, to fully im-
plement the reference architecture of DESIGN.
The Server exposes four main endpoints:
• training, which is used to train a CVS O/PDE
model. With the training application, a user may
select a specific O/PDE model from the Model
Repository, and a subset of data in the Patient
Data repository, to train the model, according to
the GOKU workflow. This service may use an-
other internal service — simulation — that could
be adopted to augment data. As in (Linial et al.,
2021), O/PDE integration can be used to gener-
ate time series — with a proper noise level — on
which Deep Learning (DL) models are trained;
• instantiate, this endpoint is used to instantiate a
new patient, with a specified set of parameter val-
ues. The Model Selector — which is responsible
for serving this endpoint — instantiates from the
Model Repository the specified model and adds
this instantiation into a Patient Cache, which con-
tains current patient models and their evolutions.
The service returns a patient id to the client;
• step, this endpoint needs the specification of the
patient id from the client. The service retrieves
the patient model from the Patient Cache and then
integrates the instantiated equations to simulate
the evolution of the x(t) Variables Vector;
• perturb, this endpoint changes the values of one
or more parameters in P , present in the Patient
Cache, according to a specified patient id.
Due to compatibility with the GOKU framework
and to the high flexibility of the language, the Python
language is a preferable choice to implement the
server.
A client ends the architecture, opening to be in-
tegrated into the Virtual Reality / Augmented Real-
ity (VR/AR) visor. The integration of VR/AR visors
will boost the impact of the DT, giving to its usage
also the capability to practically see the effects of
AI4EIoT 2025 - Special Session on Artificial Intelligence for Emerging IoT Systems: Open Challenges and Novel Perspectives
488
Server
Model
Selector
Client
Patient
Data
instantiate
Model
Learning
Patient
Cache
training
Data
Generation
Evaluator
step
Model
Repository
VR/AR visor
simulation
perturb
Model
Perturbation
Figure 4: The DESIGN microservice architecture.
Variable Vector changes. To make this solution easy
to integrate into existing VR/AR platforms — e.g.,
Unity, Unreal Engine — high-performance languages
as C#/C++ are perfect candidates to implement this
tool.
6 CONCLUSION AND FUTURE
WORK
This paper presents a reference architecture for a
CVS-DT, and an adaption of an existing prototype
for an effective implementation of such software. By
means of the proposed methods and techniques, med-
ical staff can query a model of a CVS — related to a
specific patient — and can obtain information regard-
ing the patient’s HS. The proposed architecture can
also enable what-if analysis on possible treatments
and drug administrations.
First future research efforts will be devoted to the
completion of the DESIGN tool and to the integra-
tion of real-world and simulated data. Furthermore,
the application to other human body systems is an-
other possible research task. Of course, exploring new
body mechanisms/systems and defining further vari-
ables/parameters to study, implies considering other
O/PDE models.
From the technological point of view, the building
of a full demonstrator, based on IoT sensors, capable
of interacting with the physical world as well as with
the DESIGN platform is to build. This scenario would
bring the possibility to run tests with physical subjects
(i.e., human beings and/or Human Patient Simulators
(HPSs)).
ACKNOWLEDGEMENTS
The work of Ciro Nespolino is granted by PNRR
(M4C1 – Inv. 4.1 “Pubblica amministrazione”) —
DM 118/2023 with the benefit of Universit
`
a degli
Studi della Campania “Luigi Vanvitelli”.
This work has been performed by using the com-
puting resources operated by the Department of Math-
ematics and Physics of the University of Campania
“Luigi Vanvitelli”, Caserta, Italy, within the VALERE
Program.
This work has been performed by using the Meta-
Quest 2 VR visor, kindly provided by Meta company.
REFERENCES
Alshamrani, M. (2022). IoT and artificial intelli-
gence implementations for remote healthcare moni-
toring systems: A survey. Journal of King Saud
University - Computer and Information Sciences,
34(8):4687–4701.
Antoniadi, A. M., Du, Y., Guendouz, Y., Wei, L., Mazo,
C., Becker, B. A., and Mooney, C. (2021). Current
challenges and future opportunities for xai in machine
learning-based clinical decision support systems: A
systematic review. Applied Sciences, 11(11):5088.
Campanile, L., de Biase, M. S., De Fazio, R., Di Giovanni,
M., Marulli, F., and Verde, L. (2023). Merging Model-
Based and Data-Driven Approaches for Resilient Sys-
tems Digital Twins Design. In 2023 IEEE Interna-
tional Conference on Cyber Security and Resilience
(CSR), pages 301–306. IEEE.
Chakshu, N. K., Sazonov, I., and Nithiarasu, P. (2021).
Towards enabling a cardiovascular digital twin for
human systemic circulation using inverse analy-
Towards a Digital Twin of the Cardiovascular System
489

sis. Biomechanics and modeling in mechanobiology,
20(2):449–465.
Coorey, G., Figtree, G. A., Fletcher, D. F., Snelson, V. J.,
Vernon, S. T., Winlaw, D., Grieve, S. M., McEwan,
A., Yang, J. Y. H., Qian, P., et al. (2022). The health
digital twin to tackle cardiovascular disease—a review
of an emerging interdisciplinary field. NPJ digital
medicine, 5(1):126.
De Fazio, R., Nespolino, C., Marrone, S., and Verde, L.
(2025). A unified approach for digital twins in health-
care. In Handbook of Artificial Intelligence in Health-
care, under review.
Garc
´
ıa-Moll
´
a, V. M., Liberos, A., Vidal, A., Guillem, M.,
Millet, J., Gonzalez, A., Mart
´
ınez-Zald
´
ıvar, F.-J., and
Climent, A. M. (2014). Adaptive step ode algorithms
for the 3d simulation of electric heart activity with
graphics processing units. Computers in biology and
medicine, 44:15–26.
Hassani, H., Huang, X., and MacFeely, S. (2022). Impactful
Digital Twin in the Healthcare Revolution. Big Data
and Cognitive Computing, 6(3):83.
Hemamalini, V., Armosh, F., and Tyagi, A. K. (2024). Digi-
tal Twin-Based Smart Healthcare Services for the Next
Generation Society, page 247–277. IGI Global.
Hermida, U., van Poppel, M. P., Sabry, M., Keramati,
H., Steinweg, J. K., Simpson, J. M., Vigneswaran,
T. V., Razavi, R., Pushparajah, K., Lloyd, D. F.,
et al. (2024). The onset of coarctation of the aorta
before birth: Mechanistic insights from fetal arch
anatomy and haemodynamics. Computers in Biology
and Medicine, 182:109077.
Lagana, K., Balossino, R., Migliavacca, F., Pennati, G.,
Bove, E. L., de Leval, M. R., and Dubini, G. (2005).
Multiscale modeling of the cardiovascular system: ap-
plication to the study of pulmonary and coronary per-
fusions in the univentricular circulation. Journal of
biomechanics, 38(5):1129–1141.
Linial, O., Ravid, N., Eytan, D., and Shalit, U. (2021). Gen-
erative ODE modeling with known unknowns. In Pro-
ceedings of the Conference on Health, Inference, and
Learning, pages 79–94.
Marques, L., Costa, B., Pereira, M., Silva, A., Santos, J.,
Saldanha, L., Silva, I., Magalh
˜
aes, P., Schmidt, S.,
and Vale, N. (2024). Advancing precision medicine:
A review of innovative in silico approaches for drug
development, clinical pharmacology and personalized
healthcare. Pharmaceutics, 16(3):332.
Marrone, S. (2024). What does a heart beat for? a heteroge-
neous approach for human digital twin construction.
Procedia Computer Science, 246:5132–5141.
Mulder, S. T., Omidvari, A.-H., Rueten-Budde, A. J.,
Huang, P.-H., Kim, K.-H., Bais, B., Rousian, M., Hai,
R., Akgun, C., van Lennep, J. R., et al. (2022). Dy-
namic digital twin: Diagnosis, treatment, prediction,
and prevention of disease during the life course. Jour-
nal of Medical Internet Research, 24(9):e35675.
Okegbile, S. D., Cai, J., Niyato, D., and Yi, C. (2023).
Human Digital Twin for Personalized Healthcare: Vi-
sion, Architecture and Future Directions. IEEE Net-
work, 37(2):262–269.
Quarteroni, A., Manzoni, A., and Vergara, C. (2017). The
cardiovascular system: mathematical modelling, nu-
merical algorithms and clinical applications. Acta Nu-
merica, 26:365–590.
Ramasamy, L. K., Khan, F., Shah, M., Prasad, B. V. V. S.,
Iwendi, C., and Biamba, C. (2022). Secure smart
wearable computing through artificial intelligence-
enabled internet of things and cyber-physical systems
for health monitoring. Sensors, 22(3):1076.
Romero, P., Pedr
´
os, A., Sebastian, R., Lozano, M., and
Garc
´
ıa-Fern
´
andez, I. (2025). A robust shape model
for blood vessels analysis. Applied Mathematics and
Computation, 487:129078.
Salvador, M., Strocchi, M., Regazzoni, F., Augustin, C. M.,
Dede’, L., Niederer, S. A., and Quarteroni, A. (2024).
Whole-heart electromechanical simulations using la-
tent neural ordinary differential equations. NPJ Digi-
tal Medicine, 7(1):90.
Tanade, C., Khan, N. S., Rakestraw, E., Ladd, W. D.,
Draeger, E. W., and Randles, A. (2024). Establish-
ing the longitudinal hemodynamic mapping frame-
work for wearable-driven coronary digital twins. NPJ
Digital Medicine, 7(1):236.
Wang, B., Zhou, H., Yang, G., Li, X., and Yang, H.
(2022). Human digital twin (HDT) driven human-
cyber-physical systems: Key technologies and appli-
cations. Chinese Journal of Mechanical Engineering,
35(1):11.
Yu, W., Zhao, G., Liu, Q., and Song, Y. (2021). Role of
big data analytics capability in developing integrated
hospital supply chains and operational flexibility: An
organizational information processing theory perspec-
tive. Technological Forecasting and Social Change,
163:120417.
Zenker, S., Rubin, J., and Clermont, G. (2007). From in-
verse problems in mathematical physiology to quanti-
tative differential diagnoses. PLoS computational bi-
ology, 3(11):e204.
AI4EIoT 2025 - Special Session on Artificial Intelligence for Emerging IoT Systems: Open Challenges and Novel Perspectives
490
